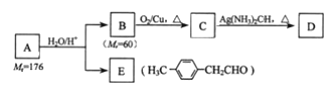
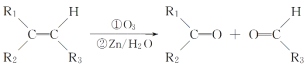
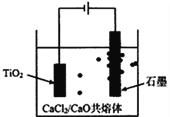
��Ŀ����
����Ŀ�����ݷ�ӦFe3+�� Ag ![]() Fe2+ �� Ag+ ������Fe3+������Һ����ʴҺ���Թ��е�����ϴȥ��
Fe2+ �� Ag+ ������Fe3+������Һ����ʴҺ���Թ��е�����ϴȥ��
��1��FeCl3��Һ�����ԣ�ԭ����_____________________________�������ӷ���ʽ��ʾ����
��2������FeCl3��Һϴ��������������ȷ����_______������ţ���
a��c��Fe3+����С��������b��c��Cl-�����䡡����c ����Ԫ��������С
��3����ͬѧ����ϴ����Һ��Fe3+�� Fe2+�� Ag+�� NO3-���л������Ϳ�ʴҺ�����������·�ߣ�

�ٹ��̢��з�Ӧ�����ӷ���ʽ��_______________________��_____________________��
�ڹ��̢��м�����Լ�������_______________��
��4���������������Һ��Ag��NH3��2OH������ʱ��������ǿ��ը�Ե����ʣ����Բ������档��������Һ�л������ķ����ǣ���������Һ�м���������ᣬ���ˣ������AgCl�м����ǰ���NH2OH������ַ�Ӧ��ɵ������ǰ�������ΪN2��
������AgCl�����Ļ�ѧ����ʽ��_____________________��
�����÷�Ӧ������3��3 g�ǰ��������Ͽɵ���������Ϊ_______g��
���𰸡�Fe3++3H2OFe��OH��3+3H+a����������NO3-Ҳ������Fe2+����������ԭ��Ӧ2Fe3++Fe=3Fe2+ Fe+2Ag+=Fe2++2Agϡ�����ϡ����Ag��NH3��2OH+3HCl=AgCl��+2NH4Cl+H2O10.8
��������
��1��Fe3+ˮ�����H+��ʹ��Һ�����ԣ�Fe3++ 3H2O![]() Fe(OH)3+3H+ ��
Fe(OH)3+3H+ ��
��2�����ݷ�Ӧ����ʽ��֪��ϴ����Fe3+ת��ΪFe2+��c��Fe3+����С����A��ȷ��B��Cl-���뷴Ӧ���ɷ�Ag+��ϳ�AgCl������c��Cl-����С����B����C����Ԫ���ڷ�Ӧǰ��ֱ���+3�ۺ�+2�۴��ڣ���������û�м��٣���C����ѡA��
��3���ٹ��̢�ӹ����з��������������˵�����̢���ϴ����Һ�м��������ۣ����û��������ʣ�����Fe3+��ԭΪFe2+��2Fe3++ Fe =3 Fe2+ ��Fe+2Ag+=Fe2++2Ag �� �ڹ��̢��м����˹������ۣ����̢����ϡ�����ϡ���ὫFeת��ΪFe2+��
��4����Ag��NH3��2OH��HCl��Ӧ����AgCl������NH4Cl��H2O������ʽΪ��Ag��NH3��2OH+3HCl=AgCl��+2NH4Cl+H2O ����2NH2OH+ 2AgCl =2Ag+N2![]() +2HCl+2 H2O������3��3 g�ǰ���0.1mol�ǰ������ɵ�������Ϊ0.1mol��������������10.8g��
+2HCl+2 H2O������3��3 g�ǰ���0.1mol�ǰ������ɵ�������Ϊ0.1mol��������������10.8g��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��NԪ����ֲ�������ı���Ԫ�أ������������ڹ�ũҵ�����Լ������ж���������Ҫ���á�
��1�������ǵ���N2O����һ��ǿ�������壬����ת���ɿ�����Ⱦ��о������ǵ��ֽ�Ի�����������Ҫ���塣��ˮ�����ѵ������У�������������£�����刺ɷֽ�ΪN2O����һ�ֲ���÷�Ӧ�Ļ�ѧ����ʽΪ____��
��2��������������ǿ�������Ҫ��Ⱦ�ͬʱҲ����Ҫ�Ļ���ԭ�ϡ�ij��ѧ����С��������Ϻ��֪2NO(g)+O2(g)![]() 2NO2(g)�ķ�Ӧ���̷�������
2NO2(g)�ķ�Ӧ���̷�������
��2NO(g)![]() N2O2(g)���죩��H1<0 K1
N2O2(g)���죩��H1<0 K1
��N2O2(g)+O2(g)![]() 2NO2(g)��������H2<0 K2
2NO2(g)��������H2<0 K2
��Ӧ2NO(g)+O2(g)![]() 2NO2(g)����H=__���ú���H1����H2��ʽ�ӱ�ʾ����K=___���ú�K1��K2��ʽ�ӱ�ʾ��������2NO(g)+O2(g)
2NO2(g)����H=__���ú���H1����H2��ʽ�ӱ�ʾ����K=___���ú�K1��K2��ʽ�ӱ�ʾ��������2NO(g)+O2(g)![]() 2NO2(g)�ķ�Ӧ���ʵ��Ƿ�Ӧ_____������ţ�����Ӧ�ٵĻ��E1���뷴Ӧ�ڵĻ��E2�Ĵ�С��ϵΪE1___������>����<������=����E2��
2NO2(g)�ķ�Ӧ���ʵ��Ƿ�Ӧ_____������ţ�����Ӧ�ٵĻ��E1���뷴Ӧ�ڵĻ��E2�Ĵ�С��ϵΪE1___������>����<������=����E2��
��3����373 Kʱ�������Ϊ2 L�ĺ������������ͨ��0.40 mol NO2��������Ӧ��2NO2(g)![]() N2O4(g)�� ��H=57.0 kJ��mol1�����NO2���������[��(NO2)]�뷴Ӧʱ��(t)�Ĺ�ϵ���±���
N2O4(g)�� ��H=57.0 kJ��mol1�����NO2���������[��(NO2)]�뷴Ӧʱ��(t)�Ĺ�ϵ���±���
t/min | 0 | 20 | 40 | 60 | 80 |
��(NO2) | 1.0 | 0.75 | 0.52 | 0.40 | 0.40 |
��0��20 min�ڣ�v(N2O4)=__________��
��������Ӧ�У�v��(NO2)=k1��c2(NO2)��v��(N2O4)=k2��c(N2O4)������k1��k2Ϊ���ʳ�������373 Kʱ��k1��k2����ѧ��ϵʽΪ____���ı��¶���T1ʱk1=k