��Ŀ����
6����֪A-K��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ���������ɵ�ˮ���������ֲ�������ȥ��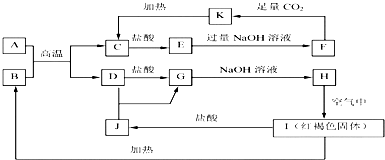
��ش��������⣺
��1��CO2 �ĵ���ʽΪ
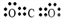 ��
����2��G��һ�������¿���ת��ΪJ����д�����ӷ���ʽ2Fe2++Cl2�T2Fe3++2Cl-��
��3����F��ͨ������CO2����K�����ӷ���ʽAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��4��E��F�����ӷ���ʽAl3++4OH-�TAlO2-+2H2O��
��5������J��Һ�������ӵķ�����ȡ������Һ���Թܣ��μ�KSCN��Һ������Һ��죬����Fe3+��
���� A��D�dz����Ľ������ʣ����ɫ����IΪFe��OH��3�����ת����ϵ��֪��BΪFe2O3����Ԫ���غ��֪��DΪFe����GΪFeCl2��HΪFe��OH��2��JΪFeCl3��A����������Ӧ����C��Fe��CΪ����������������ᡢ�������Ʒ�Ӧ������֪AΪAl��CΪAl2O3�������������ᷴӦ�õ�EΪAlCl3��EΪAlCl3���������Ʒ�Ӧ�õ�F��F�������Ķ�����̼��Ӧ�õ�K��K���ȷֽ�õ�C����FΪNaAlO2��KΪAl��OH��3���ݴ˽��
��� �⣺A��D�dz����Ľ������ʣ����ɫ����IΪFe��OH��3�����ת����ϵ��֪��BΪFe2O3����Ԫ���غ��֪��DΪFe����GΪFeCl2��HΪFe��OH��2��JΪFeCl3��A����������Ӧ����C��Fe��CΪ����������������ᡢ�������Ʒ�Ӧ������֪AΪAl��CΪAl2O3�������������ᷴӦ�õ�EΪAlCl3��EΪAlCl3���������Ʒ�Ӧ�õ�F��F�������Ķ�����̼��Ӧ�õ�K��K���ȷֽ�õ�C����FΪNaAlO2��KΪAl��OH��3��
��1��CO2Ϊ���ݻ��������ʽΪ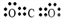 ��
��
�ʴ�Ϊ��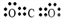 ��
��
��2���Ȼ�������ͨ�����ɵ��Ȼ�������Ӧ�����ӷ���ʽΪ2Fe2++Cl2�T2Fe3++2Cl-��
�ʴ�Ϊ��2Fe2++Cl2�T2Fe3++2Cl-��
��3����NaAlO2��ͨ������CO2����AlOH��3�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
�ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��4��EΪAlCl3��FΪNaAlO2��E��F�����ӷ���ʽΪAl3++4OH-�TAlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-�TAlO2-+2H2O��
��5��JΪFeCl3������J��Һ�������ӵķ�����ȡ������Һ���Թܣ��μ�KSCN��Һ������Һ��죬����Fe3+��
�ʴ�Ϊ��ȡ������Һ���Թܣ��μ�KSCN��Һ������Һ��죬����Fe3+��
���� ����������ͼ�ƶ��⣬���������ƶ������ʣ��ѶȽϴ�����Ԫ�ػ�����������ǽ���Ĺؼ�����Ҫѧ����������Ԫ�ػ�����֪ʶ��

| A�� | ����������Һͨ�����CO2��Ca2++2ClO-+H2O+CO2=CaCO3��+2HClO | |
| B�� | NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ�NH4++HSO3-+2OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+SO32-+2H2O | |
| C�� | MnO2��Ũ������Cl2��MnO2+4HCl$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++2Cl-+Cl2��+2H2O | |
| D�� | AlCl3��Һ�м�������İ�ˮ��Al3++4NH3•H2O=AlO2-+4NH4+ |
| A�� | ���Ӳ���ճ��Ƥ���Ͽ��þƾ�ϴ�� | |
| B�� | ���������ǻ���� | |
| C�� | ��ҵ�ƾ�ֻ����ʵ����ȼ�� | |
| D�� | ���ö���������Ի���ͭ�����ж� |
| A�� | Cl2 | B�� | HCl | C�� | SO2 | D�� | CO2 |
| A�� | N�ĵ��ʵľ������ڽ������壻U���⻯������ģ��ʾ��ͼΪ | |
| B�� | ��ҵ��V�ĵ��ʵ��Ʊ����Ե�����ڵ�NV | |
| C�� | Z������������Ӧ��ˮ������������̬�⻯�ﷴӦ������ | |
| D�� | X��Y��Z��W����Ԫ�أ���˳�����ԭ�Ӹ�����Ϊ5��1��1��3�Ļ����Ҳ���γɽṹʽΪX-W-Y��Z�Ļ����� |

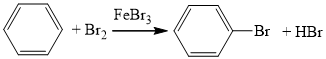 ��
�� ��
��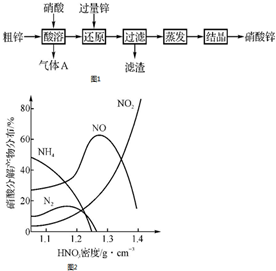 ����п�����ڹ�ҵ��ơ�ýȾ���ȣ��ô�п[������Zn2��OH��2CO3��Fe��Cu]������Ϊԭ���Ʊ�����п��ʵ��������ͼ1�����ý�������Zn��Fe��Mg�ȣ��벻ͬŨ��HNO3��Һ��Ӧʱ��Ҫ��ԭ���ﲻͬ����ͼ2��Fe�벻ͬŨ��HNO3��Һ��Ӧʱ����Ҫ��ԭ���
����п�����ڹ�ҵ��ơ�ýȾ���ȣ��ô�п[������Zn2��OH��2CO3��Fe��Cu]������Ϊԭ���Ʊ�����п��ʵ��������ͼ1�����ý�������Zn��Fe��Mg�ȣ��벻ͬŨ��HNO3��Һ��Ӧʱ��Ҫ��ԭ���ﲻͬ����ͼ2��Fe�벻ͬŨ��HNO3��Һ��Ӧʱ����Ҫ��ԭ���
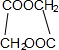 +2H2O��
+2H2O��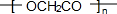 ��
��