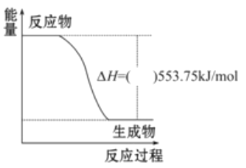
��Ŀ����
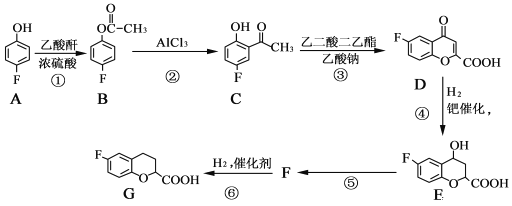
����Ŀ���α������һ������Ѫ�����ŵĽ�Ѫѹҩ�һ�ֺϳ��α�����м���G�IJ����������£�
��֪���������Ľṹ��ʽΪ![]() ��
��
��ش��������⣺
��1��A��������______��B�����������ŵ�������______��
��2����Ӧ�ݵĻ�ѧ����ʽΪ______���÷�Ӧ�ķ�Ӧ������______��
��3��G�ķ���ʽΪ______��
��4��д����������������E��ͬ���칹��Ľṹ��ʽ��______��______��
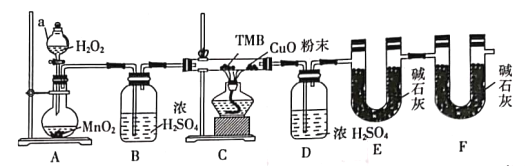
������ֻ������ȡ����
��˴Ź�������ͼ��ֻ��4�����շ�
��.1mol������������NaHCO3��Һ��Ӧ����2molCO2
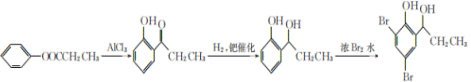
��5����������֪ʶ����������Ϣ��д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�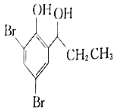 �ĺϳ�·������ͼ�����Լ���ѡ��______���ϳ�·������ͼʾ�����£�CH3CH2Br
�ĺϳ�·������ͼ�����Լ���ѡ��______���ϳ�·������ͼʾ�����£�CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3COOCH2CH3
CH3COOCH2CH3
���𰸡��Է����ӣ�4-�����ӣ� ��ԭ�ӡ����� 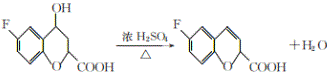 ��ȥ��Ӧ C10H9O3F
��ȥ��Ӧ C10H9O3F 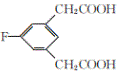
��������
(1)��ԭ�����ǻ��ڱ����Ķ�λ�ϣ�����Ϊ���Է����ӣ�4-�����ӣ���B�к��еĹ�����Ϊ��������ԭ�ӣ�
��2��ͨ���Ա�E��G��E�еĴ��ǻ���Ϊ��ԭ�ӣ����Ϊ���ǻ�����ȥ��Ӧ��
��3������G�к��б������Ȼ���֧������3��̼ԭ�ӣ������ʽΪ��C10H9O3F��
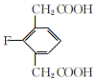
��4��E�ķ���ʽΪ��C10H9O4F�� 1mol������������ NaHCO3��Һ��Ӧ����2 mol CO2����ͬ���칹���к���2���Ȼ����˴Ź�������ͼ��ֻ��4�����շ壬������ֻ������ȡ��������ȡ����Ϊ�Գƽṹ����Ϊ�� ��
�� ��
��
��5����֪��������ˮ��Ӧ����2��4��6-���屽�ӣ���![]() ������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ��
������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ��
(1)��ԭ�����ǻ��ڱ����Ķ�λ�ϣ�����Ϊ���Է����ӣ�4-�����ӣ���B�к��еĹ�����Ϊ��������ԭ�ӣ�
��2��ͨ���Ա�E��G��E�еĴ��ǻ���Ϊ��ԭ�ӣ����Ϊ���ǻ�����ȥ��Ӧ����ѧ����ʽΪ�� ����Ӧ����Ϊ����ȥ��Ӧ��
����Ӧ����Ϊ����ȥ��Ӧ��
��3������G�к��б������Ȼ���֧������3��̼ԭ�ӣ������ʽΪ��C10H9O3F��
��4��E�ķ���ʽΪ��C10H9O4F�� 1mol������������ NaHCO3��Һ��Ӧ����2 mol CO2����ͬ���칹���к���2���Ȼ����˴Ź�������ͼ��ֻ��4�����շ壬������ֻ������ȡ��������ȡ����Ϊ�Գƽṹ����Ϊ�� ��
�� ��
��
��5����֪��������ˮ��Ӧ����2��4��6-���屽�ӣ���![]() ������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ���ʷ�Ӧ����Ϊ��
������ԭ��ǰӦ�Ƚ�������Тڵķ�Ӧ���ʷ�Ӧ����Ϊ��