��Ŀ����
����Ŀ��ClO2��Ϊһ�ֹ����͵�����������������ȡ��Cl2��Ϊ����ˮ������������֪ClO2��һ��������ˮ���������л��ܼ������壬11��ʱҺ���ɺ���ɫҺ�塣
��1��ij�о�С������ͼװ���Ʊ�����ClO2���г�װ������ȥ����
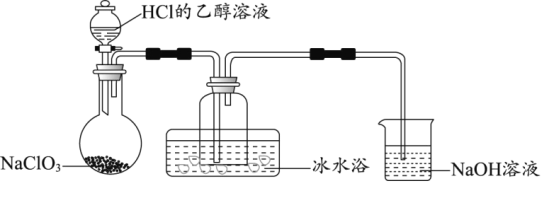
�ٱ�ˮԡ��������___��
��NaOH��Һ����Ҫ����Ϊ���շ�Ӧ������Cl2��������Һ��������ȡƯ��Һ�������շ�Ӧ�����ӷ���ʽΪ___���÷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ___��
����NaClO3��HClΪԭ���Ʊ�ClO2����ɻ�ѧ����ʽ___��
��2����ClO2ˮ��Һ�μӵ�KI��Һ�У���Һ���ػƣ��������м�������������Һ�������ã��۲쵽___��֤��ClO2���������ԡ�
��3��ClO2��ɱ�����������л����Cl-����0.0001mol��L-1��AgNO3����Һ�ζ����յ㣬���ⶨ����ˮ����Cl-�ĺ�����
���ڵζ�����װ��AgNO3����Һ��ǰһ����Ӧ���еIJ���___��
�����ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ⶨ���___������ƫ��������ƫ����������Ӱ��������
���𰸡��ռ�ClO2����ʹClO2����ΪҺ�壩 Cl2+OH-=Cl-+ClO-+H2O 1��1 2NaClO3+4HCl=2ClO2��+ Cl2��+2NaCl+2H2O ��Һ��Ϊ��ɫ ��AgNO3����Һ������ϴ ƫ��
��������
(1)�ٱ�ˮԡ�ɽ����¶ȣ���ֹ�ӷ���
���������������Ʒ�Ӧ�����Ȼ��ơ��������ƣ�
����NaClO3��HClΪԭ���Ʊ�ClO2��ͬʱ��Cl2��NaCl���ɣ���ϵ����غ��ԭ���غ�д��������Ӧ�Ļ�ѧ����ʽ��
(2)��Ӧ���ɵ⣬�����������Ȼ�̼���²�Ϊ��ɫ��
(3)���ݵζ���������
(1)�ٱ�ˮԡ�ɽ����¶ȣ���ֹ�ӷ����������������ռ�ClO2���ʴ�Ϊ���ռ�ClO2(��ʹClO2����ΪҺ��)��
���������������Ʒ�Ӧ�����Ȼ��ơ��������ƣ���Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaCl+NaClO+H2O�������ӷ���ʽΪ��Cl2+OH-=Cl-+ClO-+H2O���˷�Ӧ��Cl2���������������ǻ�ԭ������ԭ����NaCl����������NaClO�����ʵ���֮��Ϊ1:1��������շ�Ӧ���������뻹ԭ��֮��Ϊ1:1���ʴ�Ϊ��Cl2+OH-=Cl-+ClO-+H2O��1��1��
����NaClO3��HClΪԭ���Ʊ�ClO2��ͬʱ��Cl2��NaCl���ɣ�������Ӧ�Ļ�ѧ����ʽΪ2NaClO3 + 4HCl= 2ClO2��+ Cl2��+2NaCl+2H2O���ʴ�Ϊ��2NaClO3+4HCl=2ClO2��+ Cl2��+2NaCl+2H2O��
(2)��ClO2ˮ��Һ�μӵ�KI��Һ�У���Һ���ػƣ�˵�����ɵ⣬�����������Ȼ�̼�������۲쵽��Һ�ֲ㣬�²�Ϊ�Ϻ�ɫʱ��˵����I2���ɣ�����ClO2���������ԣ��ʴ�Ϊ����Һ��Ϊ��ɫ��
(3)��װ��AgNO3����Һ��Ӧ����Ũ�Ƚ��ͣ�Ӧ��AgNO3����Һ������ϴ����װ��AgNO3����Һ���ʴ�Ϊ����AgNO3����Һ������ϴ��
���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬�������ı�Һ���������ƫС����ⶨ���ƫ�ͣ��ʴ�Ϊ��ƫ�͡�

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�����Ŀ�����û�ѧ��Ӧԭ���о�̼��������ĵ��ʼ��仯����ķ�Ӧ�Ի������Ⱦ����ԴΣ��������Ҫ���塣
��1��CO��ԭNO�ķ�ӦΪ2CO(g)+2NO(g)![]() 2CO2(g)+N2(g)H=-746kJ��mol-1��
2CO2(g)+N2(g)H=-746kJ��mol-1��
д���������������NOƽ��ת���ʵĴ�ʩ______________��______________��
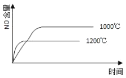
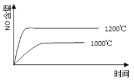
��2���ý�̿��ԭNO�ķ�ӦΪ��2NO(g)+C(s)![]() N2(g)+CO2(g)
N2(g)+CO2(g)
H�����ݺ��������£��������ͬ�ļס��ҡ������������зֱ���������Ľ�̿��һ������NO����ø�������NO�����ʵ���[n(NO)]�淴Ӧʱ�䣨t���ı仯��������ʾ��
t/min n(NO)/mol ���� | 0 | 40 | 80 | 120 | 160 |
��/400�� | 2.00 | 1.5 | 1.10 | 0.80 | 0.80 |
��/400�� | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
��/T�� | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
��H______________0����������������������
����������160minʱ��v��_________v������������������������=������
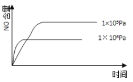
��3��ij�¶��£������Ϊ2L�ĺ������������ͨ��2.0molNO2��������Ӧ��2NO2(g)![]() N2O4(g)H=-57.0kJ��mol-1����֪��v��(NO2)=k1��c2(NO2)��v��(N2O4)=k2��c(N2O4)������k1��k2Ϊ���ʳ��������NO2���������[x(NO2)]�뷴Ӧʱ�䣨t���Ĺ�ϵ�����
N2O4(g)H=-57.0kJ��mol-1����֪��v��(NO2)=k1��c2(NO2)��v��(N2O4)=k2��c(N2O4)������k1��k2Ϊ���ʳ��������NO2���������[x(NO2)]�뷴Ӧʱ�䣨t���Ĺ�ϵ�����
t/min | 0 | 20 | 40 | 60 | 80 |
x(NO2) | 1.0 | 0.75 | 0.52 | 0.50 | 0.50 |
��![]() ����ֵΪ______________��
����ֵΪ______________��
����֪���ʳ���k���¶����߶������������¶Ⱥ�k1����ı���___________k2����ı�������������������������=������
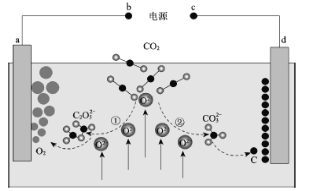
��4���ü�ӵ绯ѧ����ȥNO�Ĺ��̣���ͼ��ʾ��
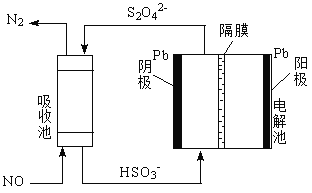
����֪���ص�����������Һ��pH��4~7֮�䣬д�������ĵ缫��Ӧʽ��______________��
�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ����______________��