��Ŀ����
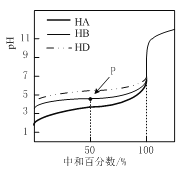
����Ŀ�������£���ijһԪ��HX��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
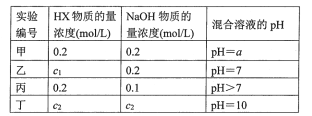
��ش�
(1)�������������ʵ���������Ӽ��������������a ___7(����>""<������=��)����HXΪǿ���a_________7������>����<������=��������HXΪ���ᣮ
(2)����������Һ������Ũ��c(X-)��c(Na+)�Ĵ�С��ϵ��____��
A.ǰ�ߴ� B�����ߴ� C��������� D�����ж�
(3)�ӱ���ʵ����������HX��___ �ᣨ����ǿ���������������û����Һ������Ũ���ɴ�С��˳����________��
(4)����ʵ�����û����Һ����ˮ�������c(OH-)=_________mol/L.
���𰸡�= > C �� c(Na��)>c(X��)>c(OH��)>c(H��) 10��4
��������
��1��������ҺŨ����ͬ�������ͬ��ǡ����ȫ��Ӧ������ΪNaX�����pH=7��X��������ˮ�⣬��˵��HXΪǿ�ᣬ���pH>7��X������ˮ�⣬��˵��HXΪ���
��2�����ݵ���غ㣬c(Na��)��c(H��)=c(OH��)��c(X��)��pH=7��ˮ��Һ��c(H��)=c(OH��)������c(Na��)=c(X��)����C��ȷ��
��3���������ϣ���Ӧ������ΪNaX��HX����ΪpH>7����Һ�Լ��ԣ�X������ˮ�⣬��HXΪ���������Һ��Ӧ��NaX��HX�����ʵ�����ȣ���X������ˮ�⣺X����H2O![]() HX��OH����ˮ��̶����������Ӵ�С˳����c(Na��)>c(X��)>c(OH��)>c(H��)��
HX��OH����ˮ��̶����������Ӵ�С˳����c(Na��)>c(X��)>c(OH��)>c(H��)��
��4�����飺���ʵ���Ũ����ͬ���������Ϻ�ǡ����ȫ��Ӧ������NaX����Һ��pH=10����ˮ�����(OH��)=10��4mol��L��1��