��Ŀ����
��1�����С�H��ʾ����ȼ���ȵ���
A��2H2��g��+O2��g���T2H2O��l����H1
B��C��s��+1/2O2��g���TCO��g����H2
C��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H3
D��C��s��+O2��g���TCO2��g����H4
E��C6H12O6��s��+6O2��g���T6CO2��g��+6H2O��l����H5
F��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H6
G��2NaOH��aq��+H2SO4��aq���TNa2SO4��aq��+2H2O��l����H7
H��CH3COOH��aq��+NaOH��aq���TCH3COONa��aq��+H2O��l����H8
��2��2.00g C2H2������ȫȼ������Һ̬ˮ��CO2���壬�ų�99.6kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ
| 5 |
| 2 |
| 5 |
| 2 |
��3�����ȼ��һ�����Ķ���ų���������СΪQ�����ɵ�CO2ǡ����100mLŨ��Ϊ5mol?L-1��KOH��Һ��ȫ��Ӧ����һ�����Σ���ȼ��1mol����ų�������Ϊ
��2��CO2ǡ����KOH��Һ��ȫ��Ӧ��������ΪK2CO3������n=cV����KOH�����ʵ��������ݼ�Ԫ���غ����n��K2CO3��������̼Ԫ���غ���n��CO2��=n��K2CO3�����������㶡������ʵ������ݴ˼��㣮
B��̼ȼ�յ�������ΪCO����ʾ�¶ȵ�������CO2����Ӧ�ȡ�H2���ܱ�ʾȼ���ȣ�
C��1mol������ȫȼ�գ����ɵ�ˮ����̬�������ȶ���״̬��ӦΪҺ̬ˮ���ʷ�Ӧ�ȡ�H3���ܱ�ʾȼ���ȣ�
D��C��s��+O2��g���TCO2��g����H4��1molC��ȫȼ�����ɶ�����̼������ȼ���ȸ����Ӧ�ȡ�H4�ܱ�ʾȼ���ȣ�
E��C6H12O6��s��+6O2��g���T6CO2��g��+6H2O��l����H5��1molC6H12O6��ȫȼ�����ɶ�����̼��Һ̬ˮ������ȼ���ȸ����Ӧ�ȡ�H5�ܱ�ʾȼ���ȣ�
F��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H6��ʵ����1mol��������1mol���������ӷ�Ӧ����1molH2O�������к��ȸ����Ӧ�ȡ�H6�ܱ�ʾ�к��ȣ�
G����Ӧ���ɵ�ˮΪ2mol����Ӧ�ȡ�H7���ܱ�ʾ�к��ȣ�
H��CH3COOH��aq��+NaOH��aq���TCH3COONa��aq��+H2O��l����H8������Ӧ����1molH2O�������к��ȸ����Ӧ�ȡ�H8�ܱ�ʾ�к��ȣ�
�ʴ�Ϊ����H4����H5����H6����H8��
��2��2.00g C2H2������ȫȼ������Һ̬ˮ��CO2���壬�ų�99.6kJ����������1molC2H2����������ȫȼ�շų�������Ϊ
| 1mol��26g/mol |
| 2g |
| 5 |
| 2 |
�ʴ�Ϊ��C2H2��g��+
| 5 |
| 2 |
��3��KOH�����ʵ���Ϊ0.1L��5mol/L=0.5mol�����ݼ������غ㣬��n��K2CO3��=0.5mol��
| 1 |
| 2 |
| 1 |
| 4 |
| 1 |
| 16 |
| 1 |
| 16 |
| 1mol | ||
|
�ʴ�Ϊ��16QkJ��

(12��)(2011�������߶����)��ѧ��Ӧ�����з������ʱ仯��ͬʱ���������������ı仯�����������ı仯�������ܵ���ʽ���ֳ�����������Ӧ�ȡ����ڷ�Ӧ�������ͬ����Ӧ�ȿ��Է�Ϊ�����֣���ȼ���Ⱥ��к��ȵȡ�
(1)���Ц�H��ʾ����ȼ���ȵ���________����ʾ�����к��ȵ���________��(�H1����H2�ͦ�H3��)
| A��2H2(g)��O2(g)===2H2O(l)����H1 |
| B��C(s)��O2(g)===CO(g)����H2 |
| C��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H3 |
| D��C(s)��O2(g)===CO2(g)����H4 |
F��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H6
G��2NaOH(aq)��H2SO4(aq)===Na2SO4(aq)��2H2O(l)����H7
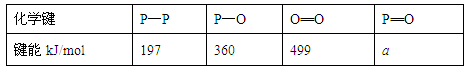
(2)��һ���о���������ѧ��Ӧ�������仯(��H)�뷴Ӧ���������ļ����йء����ܿ��Լ�����Ϊ�Ͽ�1 mol��ѧ��ʱ�������յ��������±��Dz��ֻ�ѧ���ļ������ݣ�
| ��ѧ�� | P��P | P��O | O===O | P===O |
| ����kJ/mol | 197 | 360 | 499 | x |

(12��)(2011�������߶����)��ѧ��Ӧ�����з������ʱ仯��ͬʱ���������������ı仯�����������ı仯�������ܵ���ʽ���ֳ�����������Ӧ�ȡ����ڷ�Ӧ�������ͬ����Ӧ�ȿ��Է�Ϊ�����֣���ȼ���Ⱥ��к��ȵȡ�
(1)���Ц�H��ʾ����ȼ���ȵ���________����ʾ�����к��ȵ���________��(�H1����H2�ͦ�H3��)
A��2H2(g)��O2(g)===2H2O(l)����H1
B��C(s)��O2(g)===CO(g)����H2
C��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H3
D��C(s)��O2(g)===CO2(g)����H4
E��C6H12O6(s)��6O2(g)===6CO2(g)��6H2O(l)����H5
F��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H6
G��2NaOH(aq)��H2SO4(aq)===Na2SO4(aq)��2H2O(l)����H7
(2)��һ���о���������ѧ��Ӧ�������仯(��H)�뷴Ӧ���������ļ����йء����ܿ��Լ�����Ϊ�Ͽ�1 mol��ѧ��ʱ�������յ��������±��Dz��ֻ�ѧ���ļ������ݣ�
|
��ѧ�� |
P��P |
P��O |
O===O |
P===O |
|
����kJ/mol |
197 |
360 |
499 |
x |
��֪����(P4)��ȼ����Ϊ2 378.0 kJ/mol��������ȫȼ�յIJ���(P4O10)�Ľṹ��ͼ��ʾ�����ϱ���x��________��