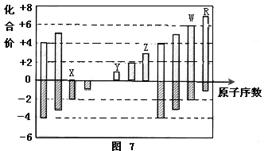
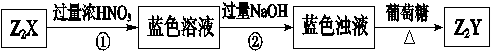��Ŀ����
�±�ΪԪ�����ڱ���һ���֣�
��1��д��Ԫ�آ������ڱ��е�λ��______________��
��2���ڡ��ۡ��ݵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��3���ܡ��ݡ�����̬�⻯����ȶ�����ǿ������˳����_________________________��
��4���١��ڡ��ۡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
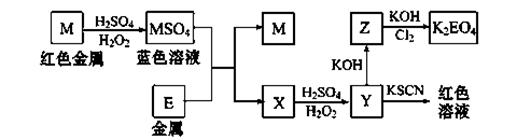
��.����������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ��ʾ�ı仯������A��һ�ֵ���ɫ���塣��ش�

��1��д������A��Һ��X��Ӧ�����ӷ���ʽ ��
��2������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1:1��ǡ����ȫ��Ӧʱ��������ҺD������Ϊ ���ѧʽ������֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ
��3��д������C������Y��Ӧ�Ļ�ѧ����ʽ ��
| �� ���� | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
��1��д��Ԫ�آ������ڱ��е�λ��______________��
��2���ڡ��ۡ��ݵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_________________________��
��3���ܡ��ݡ�����̬�⻯����ȶ�����ǿ������˳����_________________________��
��4���١��ڡ��ۡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��.����������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ��ʾ�ı仯������A��һ�ֵ���ɫ���塣��ش�

��1��д������A��Һ��X��Ӧ�����ӷ���ʽ ��
��2������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1:1��ǡ����ȫ��Ӧʱ��������ҺD������Ϊ ���ѧʽ������֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ
��3��д������C������Y��Ӧ�Ļ�ѧ����ʽ ��
��14�֣�
��1���������ڵڢ�A�壨1�֣�
��2��Na��S��O��2�֣�
��3��HCl��H2S��SiH4��2�֣�
��4��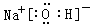 ��
��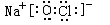 ��2�֣�
��2�֣�
��1��2Na2O2 +2H2O = 4Na+ + 4OH�D + O2����2�֣�
��2��NaHSO3��1�֣�
c(Na��)> c(HSO3��) > c(H��) > c(SO32��) > (OH��)��2�֣�
��3��2SO2 +O2 2SO3��2�֣�
2SO3��2�֣�
��1���������ڵڢ�A�壨1�֣�
��2��Na��S��O��2�֣�
��3��HCl��H2S��SiH4��2�֣�
��4��
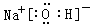 ��
��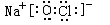 ��2�֣�
��2�֣���1��2Na2O2 +2H2O = 4Na+ + 4OH�D + O2����2�֣�
��2��NaHSO3��1�֣�
c(Na��)> c(HSO3��) > c(H��) > c(SO32��) > (OH��)��2�֣�
��3��2SO2 +O2
 2SO3��2�֣�
2SO3��2�֣��������������Ԫ���������ڱ��е�λ�ã���֪Ԫ�ص����࣬��ΪHԪ�أ���ΪOԪ�أ���ΪNaԪ�أ���ΪSiԪ�أ���ΪSԪ�أ���ΪClԪ�أ�
�Ţ�ΪSiԪ��,�����ڱ��е�λ�õ������ڵڢ�A��;�𰸣��������ڵڢ�A�塣
�Ƶ��Ӳ�Խ��뾶Խ���Ӳ���һ���ģ�������ԽС���뾶Խ��ΪNaԪ�أ���ΪSԪ�أ�ͬһ���ڣ�Na��S����ΪOԪ�أ���ΪSԪ�أ�ͬһ���壬S��O���ڡ��ۡ��ݵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��Na��S��O���𰸣�Na��S��O��
��ͬ����Ԫ�ش�����Ԫ�صķǽ���������ǿ��ͬ����Ԫ�ش��ϵ���Ԫ�صķǽ�����������Ԫ�صķǽ�����Խǿ����Ӧ�⻯��Խ�ȶ���Cl��S��Si,ͬһ���ڣ����Ԣܡ��ݡ�����̬�⻯����ȶ�����ǿ������˳���ǣ�HCl��H2S��SiH4���𰸣�HCl��H2S��SiH4��
��H��O��Na��Cl�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ������ж��֣��磺NaOH��NaClO�ȣ�����ʽΪ
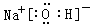 ��
��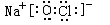 ���𰸣�
���𰸣�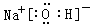 ��
��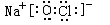 ��
����A��һ�ֵ���ɫ���壬A��Na2O2������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����꣬YΪSO2���Ƴ���A��Na2O2 B��NaOH C��O2 D��NaClO E��SO3 F��H2SO4 X��H2O Y��SO2
��1��Na2O2��ˮ��Ӧ��2Na2O2 +2H2O = 4Na+ + 4OH�D + O2���𰸣�2Na2O2 +2H2O = 4Na+ + 4OH�D + O2����
��2����NaOH��SO2���ʵ���֮��Ϊ1:1��ǡ����ȫ��Ӧʱ��NaOH��SO2=NaHSO3,������ҺD������ΪNaHSO3,HSO3�D�ĵ������ˮ�⣬������Һ�����ԣ���Һ�и�������Ũ���ɴ�С��˳��Ϊ��c(Na��)> c(HSO3��) > c(H��) > c(SO32��) > (OH��)���𰸣�NaHSO3��c(Na��)> c(HSO3��) > c(H��) > c(SO32��) > (OH��)
��3������SO2������O2��Ӧ�Ļ�ѧ����ʽ ;2SO2 +O2
 2SO3���𰸣�2SO2 +O2
2SO3���𰸣�2SO2 +O2 2SO3��
2SO3��
��ϰ��ϵ�д�
�����Ŀ