��Ŀ����
��һ���㡢Ϻ������С�ӱ��������������ס��ҡ�����������ͼ��ʾ���������ų��ķ�Һ�ÿ������ֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壬�ܶ����������ݣ���ˮ���壬��M��ˮ����pHС��7�����жϣ�
��1�����������ų��ķ�Һ�ﺬ�е���Ⱦ�
��______����______��
��______����______��
��2��д���ҡ�������������Ӧ�����ӷ���ʽ����______����______��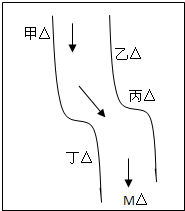
��1�����������ų��ķ�Һ�ﺬ�е���Ⱦ�
��______����______��
��______����______��
��2��д���ҡ�������������Ӧ�����ӷ���ʽ����______����______��

�ɼ״���ˮ�����ɫ�����ǿɵ��ڼ״�Ӧ����Ca��OH��2��
�Ҵ���ˮ�ʺ��ɫ�����ǿ�֪���ڼ״����·������ŷų�����FeCl3��
������ˮ�ɻ���壬���ǿɵñ����ڼ��ҵ��·��ұ����ŷų�����HCl��
�����������ݣ���ˮ���壬˵�����ڱ����·��Һ���̼���ƣ�
M�ڹ������·�������M���ĺ�ˮ�п϶����е������У�Na+��Cl-��Ca2+��
�ʴ�Ϊ��Ca��OH��2��FeCl3��HCl��Na2CO3��
��2�����Ҵ������Ȼ�������������֮��ķ�Ӧ��ʵ���ǣ�Fe3++3OH-=Fe��OH��3���ڶ������������̼����֮��ķ�Ӧ��ʵ���ǣ�CO32-+2H+�TCO2��+H2O��
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3��CO32-+2H+�TCO2��+H2O��
�Ҵ���ˮ�ʺ��ɫ�����ǿ�֪���ڼ״����·������ŷų�����FeCl3��
������ˮ�ɻ���壬���ǿɵñ����ڼ��ҵ��·��ұ����ŷų�����HCl��
�����������ݣ���ˮ���壬˵�����ڱ����·��Һ���̼���ƣ�
M�ڹ������·�������M���ĺ�ˮ�п϶����е������У�Na+��Cl-��Ca2+��
�ʴ�Ϊ��Ca��OH��2��FeCl3��HCl��Na2CO3��
��2�����Ҵ������Ȼ�������������֮��ķ�Ӧ��ʵ���ǣ�Fe3++3OH-=Fe��OH��3���ڶ������������̼����֮��ķ�Ӧ��ʵ���ǣ�CO32-+2H+�TCO2��+H2O��
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3��CO32-+2H+�TCO2��+H2O��

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
|
��1�����������ų��ķ�Һ�ﺬ�е���Ⱦ�
�� ���� ���� ���� ��
��2����M��ȡ���ĺ�ˮ�У��϶����е�������
��
��3��С������Ϻ����������ԭ����
���������������� ��������
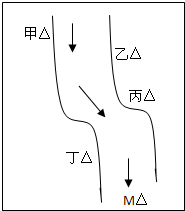 ��һ���㡢Ϻ������С�ӱ��������������ס��ҡ�����������ͼ��ʾ���������ų��ķ�Һ�ÿ������ֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壬�ܶ����������ݣ���ˮ���壬��M��ˮ����pHС��7�����жϣ�
��һ���㡢Ϻ������С�ӱ��������������ס��ҡ�����������ͼ��ʾ���������ų��ķ�Һ�ÿ������ֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壬�ܶ����������ݣ���ˮ���壬��M��ˮ����pHС��7�����жϣ�
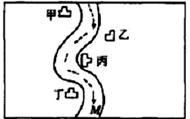
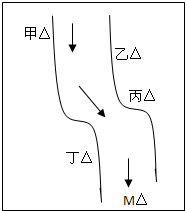 ��һ���㡢Ϻ������С�ӱ��������������ס��ҡ�����������ͼ��ʾ���������ų��ķ�Һ�ÿ������ֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壬�ܶ����������ݣ���ˮ���壬��M��ˮ����pHС��7�����жϣ�
��һ���㡢Ϻ������С�ӱ��������������ס��ҡ�����������ͼ��ʾ���������ų��ķ�Һ�ÿ������ֻ����Na2CO3��FeCl3��Ca��OH��2��HCl�е�һ�֣�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壬�ܶ����������ݣ���ˮ���壬��M��ˮ����pHС��7�����жϣ�