��Ŀ����
I��ij��ѧ����С�����Ⱦ�����IJ��ַǽ������������̽�����������ĿҪ��ش��������⣮
��1��д����������ȡһ�����������ӷ���ʽ______��
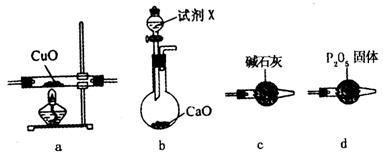
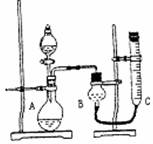
��2�������ϵ�֪��HCOOH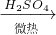 CO��+H2O��ʵ��������ͼ1��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ______������ţ���ʵ�������ø�װ�û�����ȡ�ij���������______����дһ������ķ���ʽ����
CO��+H2O��ʵ��������ͼ1��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��Ϊ______������ţ���ʵ�������ø�װ�û�����ȡ�ij���������______����дһ������ķ���ʽ����
��3�������ϵ�֪�����ô�����ʹ����β���е�һ����̼�͵�������ַ�����Ӧת��Ϊ������̼�͵�������С����ʵ����ģ������β���������������ͼ2��ʾװ�ã����ּгֺ�װ������ȥ����
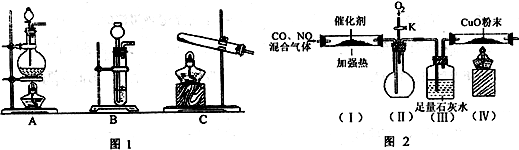
��ʵ��ǰ�ر�����K����ͨ�����ž�װ���еĿ�������Ŀ����______��
��װ�ã�����Ҫ������______��
�۸���װ�����в�����֮������Ӧ��װ�ã�������______װ�ã�
II���ÿ���С����ƵĴ�ZnSO4��FeCl3�Ļ����Һ����ȡZnSO4?7H2O�������£�
a���ڻ��Һ�м���6mol?L-1 NaOH��Һ����pH=8Ϊֹ��
b�����˺�õ�������������ˮ���ϴ�ӳ�����
c����ϴ�Ӻ�ij����м���2mol?L-1�����ᣬ������Һ��pH��4��6��������У����ȹ��ˣ���Һ��ΪZnSO4��Һ��
d����Һ�м���2mol?L-1�����ᣬʹ��pH=2��
��֪�����������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���±����ش��������⣺
| ������ | Fe��OH��3 | Zn��OH��2 |
| pH | 1.5��3.2 | 6.4��8.0 |
��2������d�м������ᣬʹ��pH=2��Ŀ����______�� Ҫ�Ƶ�ZnSO4?7H2O�IJ���d��ȱ�ٵIJ�����______�����õ���Ҫ������������______��
��2���÷�ӦΪҺ�����װ�ã���Ũ����Ͷ������̼��ȿ���ȡ�������ʴ�Ϊ��A��Cl2��
��3���ٷ�ֹ��ȼ�������������������Ϸ�����ը���ʴ�Ϊ����ֹCO�������ϼ��ȱ�ը��
��CO2�������ʯ��ˮ����ǣ���һ����̼���ܣ��ʴ�Ϊ������CO�Ƿ�ת��ΪCO2��
��һ����̼�ж�������Ҫע���ֹһ����̼й©�Ӷ���Ⱦ�������ʴ�Ϊ��β������װ�ã�
II����1��ȡ���һ��ϴ��Һ�������μ���������Һ�����ް�ɫ������˵���Ѿ�ϴ�Ӹɾ����ʴ�Ϊ��ȡ���һ��ϴ��Һ�������μ���������Һ�����ް�ɫ������˵���Ѿ�ϴ�Ӹɾ���
��2����ΪZn2+pHΪ6.4��8.0ʱ�ɷ���ˮ�⣻Ҫ�Ƶ�ZnSO4?7H2O����Ҫ����Һ����Ũ������ȴ�ᾧ������������Ҫ�����������ƾ��ƣ����������ʴ�Ϊ������Zn2+��ˮ�⣻����Һ����Ũ������ȴ�ᾧ������������Ҫ�������ƾ��ƣ���������
������I����1����ͭмϡ����Ϊԭ����ȡ����ͭͬʱ������һ��������
��2��ѡ������ķ���װ�ø��ݷ�Ӧ���״̬�ͷ�Ӧ������
��3���ٿ�ȼ�������ڵ�ȼǰ����Ҫ�鴿����ֹ������ը��
��CO2�������ʯ��ˮ����ǣ�
�۸���һ����̼�ж����з�����
II����1��ȡ���һ��ϴ��Һ�������μ���������Һ�����ް�ɫ������˵���Ѿ�ϴ�Ӹɾ���
��2��Zn2+pHΪ6.4��8.0ʱ�ɷ���ˮ�⣻Ҫ�Ƶ�ZnSO4?7H2O����Ҫ����Һ����Ũ������ȴ�ᾧ������������Ҫ�������ƾ��ƣ���������
���������⿼��֪ʶ�ۺ϶�ȫ�棬�ܺܺõĿ����ѧ������ѧ֪ʶ�����պ�Ӧ�������

ij��ѧ����С��Ϊ��̽����ͬ������������ֽ����ʵ�Ӱ�죬��Ʋ�����������ʵ�飬����������и�������
I������ͭ���Ʊ���
��1����ȡ10g�������������С�ձ��У�����������ˮ�����Һ��
��2�����裨1���е��ձ��ڵμ�NaOH��Һ��ֱ�����������ij�����
��3����������Ƿ���ȫ������������� ��
��4�������裨2�����û�������������ȫ����Ϊ��ɫ��
��5���ٽ����裨4�����û���� ��ϴ�ӡ� ����ϸ����������Ҫ��֤�Ƿ�ϴ����������ӵķ����ǣ� ��
II���Ƚϲ�ͬ�����Թ�������ֽ����ʵ�Ӱ�졣
�ÿ���С������ɵ�ʵ�鼰ʵ���¼�ı������£�
�������ͼ��ʾװ�����ⶨ�����������
| ʵ����� | ˫��ˮ��� | ���� | �������� |
| �� | 15mL | �� | |
| �� | 15mL | CuO(0.5g) | |
| �� | 15mL | MnO2(0.5g) |
�Իش��������⣺
��1����ʵ����Ӱ��˫��ˮ�ֽ����ʵ������У���ʵ��ʱ���¶Ⱥ�ѹǿ����˫��ˮ��Ũ��
�۲�ͬ�Ĵ������� ���� �ȡ�
��2������ʵ���еġ��������ݡ������� ��Ҳ������ ��
��3��Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�飨����д�������������֤��CuO�Ļ�ѧ����û�иı䣻�� ��
��4������Ϊ����ʹ��������ֽ�Ĵ������� ����һ�����ʵĻ�ѧʽ�����ƣ���