题目内容
【题目】氮和硫的化合物在工农业生产、生活中具有重要应用。请回答下列问题:
(1)航天领域中常用N2H4作为火箭发射的助燃剂。N2H4与氨气相似,是一种碱性气体,易溶于水,生成弱碱N2H4·H2O。用电离方程式表示N2H4·H2O显碱性的原因是:____________________________________________。
(2)在恒温条件下,1 mol NO2和足量C发生反应2NO2(g)+2C(s)![]() N2(g)+2CO2(g),测得平衡时NO2和CO2的物质的量浓度与平衡总压的关系如图所示:
N2(g)+2CO2(g),测得平衡时NO2和CO2的物质的量浓度与平衡总压的关系如图所示:

①A、B两点的浓度平衡常数关系:Kc(A) ___________Kc(B)(填“<”或“>”或“=”)
②A、B、C三点中NO2的转化率最高的是___________(填“A”或“B”或“C”)点。
③计算C点时该反应的压强平衡常数Kp=___________MPa(Kp是用平衡分压代替平衡浓度计算,分压=总压×物质的量分数)。
(3)已知:亚硝酸(HNO2)性质和硝酸类似,但它是一种弱酸。常温下亚硝酸的电离平衡常数Ka=5.1×10-4;H2CO3的Ka1=4.2×10-7,Ka2=5.61×10-11。在常温下向含有2mol碳酸钠的溶液中加入1mol的HNO2后,则溶液中CO32-、HCO3-和NO2-的离子浓度由大到小的顺序是______________________。
(4)已知:常温下甲胺(CH3NH2)的电离常数为Kb,且pKb=-lgKb=3.4水溶液中有CH3NH2+H2OCH3NH3++OH-。常温下向CH3NH2溶液滴加稀硫酸至c(CH3NH2)=c(CH3NH3+)时,则溶液pH=_______。
(5)一种煤炭脱硫技术可以把硫元素以CaSO4的形成固定下来,但产生的CO又会与CaSO4发生化学反应,相关的热化学方程式如下:
①CaSO4(s)+CO(g) CaO(s)+SO2(g)+CO2(g) △H=+210.5kJ· mol-1
②CaSO4(s)+4CO(g) CaS(s)+ 4CO2(g) △H=-189.2 kJ· mol-1
反应CaO(s)+3CO(g)+SO2(g) CaS(s)+3CO2(g) △H=___________ kJ· mol-1
【答案】N2H4·H2O![]() N2H5++OH-(或N2H4+H2O
N2H5++OH-(或N2H4+H2O![]() N2H5++OH-) = A 2 c(HCO3-)>c(NO2-)>c(CO32-) 10.6 -399.7
N2H5++OH-) = A 2 c(HCO3-)>c(NO2-)>c(CO32-) 10.6 -399.7
【解析】
(1) N2H4结合H2O电离的氢离子使溶液呈碱性;
(2)①平衡常数只受温度影响,据此判断;
②增大压强平衡向气体体积减小的方向移动;
③在C点时,二氧化碳与二氧化氮的浓度相等,根据反应2NO2(g)+2C(s)![]() N2(g)+2CO2(g),可以知道氮气的浓度为二氧化碳的一半,据此确定各成份气体的体积分数,进而确定反应的压强平衡常数Kp;
N2(g)+2CO2(g),可以知道氮气的浓度为二氧化碳的一半,据此确定各成份气体的体积分数,进而确定反应的压强平衡常数Kp;
(3)弱酸电离常数越大,酸性越强,其酸根离子水解程度越小;
(4) 根据Kb=![]() =c(OH-)计算;
=c(OH-)计算;
(5) 利用盖斯定律将②-①可得CaO(s)+3CO(g)+SO2(g) CaS(s)+3CO2(g)反应热。
(1)肼易溶于水,它是与氨类似的弱碱,则电离生成OH-和阳离子,电离方程式为N2H4·H2O![]() N2H5++OH-,
N2H5++OH-,
因此,本题正确答案是:N2H4·H2O![]() N2H5++OH-;
N2H5++OH-;
(2)①平衡常数只受温度影响,所以Kc(A) =Kc(B),
因此,本题正确答案是:=;
②增大压强平衡向气体体积减小的方向移动,该反应的正反应是气体体积增大的反应,所以A、B、C三点中NO2的转化率最高的是A点,
因此,本题正确答案是:A;
③在C点时,二氧化碳与二氧化氮的浓度相等,根据反应2NO2(g)+2C(s)![]() N2(g)+2CO2(g),可以知道氮气的浓度为二氧化碳的一半,所以混合气体中CO2的体积分数为
N2(g)+2CO2(g),可以知道氮气的浓度为二氧化碳的一半,所以混合气体中CO2的体积分数为![]() ,NO2的体积分数
,NO2的体积分数![]() ,N2的体积分数
,N2的体积分数![]() ,所以反应的压强平衡常数Kp=
,所以反应的压强平衡常数Kp=![]() =
=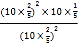 =2MPa,
=2MPa,
因此,本题正确答案是:2;
(3)2mol碳酸钠的溶液中加入1mol的HNO2后,得到含有1mol碳酸钠、1mol碳酸氢钠和1mol亚硝酸钠的混合液。由亚硝酸的电离平衡常数Ka=5.1×10-4,H2CO3的Ka1=4.2×10-7,Ka2=5.61×10-11,可判断水解程度大小顺序为CO32-> NO2-,CO32-水解生成HCO3-,水解的程度较小,所以溶液中CO32-离子、HCO3-离子和NO2-离子的浓度大小关系为c(HCO3-)>c(NO2-)>c(CO32-),
因此,本题正确答案是:c(HCO3-)>c(NO2->c(CO32-);
(4)电离常数Kb只随温度改变,常温时,CH3NH2+H2OCH3NH3++OH-,当滴加稀硫酸至c(CH3NH2)=c(CH3NH3+)时,Kb=![]() =c(OH-),即c(OH-)= Kb=10-3.4mol/L,则c(H+)=
=c(OH-),即c(OH-)= Kb=10-3.4mol/L,则c(H+)=![]() =10-10.6,所以pH=-lg10-10.6=10.6,
=10-10.6,所以pH=-lg10-10.6=10.6,
因此,本题正确答案是:10.6;
(5) 利用盖斯定律将②-①可得CaO(s)+3CO(g)+SO2(g) CaS(s)+3CO2(g)
△H=(-189.2 kJ· mol-1)-(+210.5kJ· mol-1)=-399.7 kJ· mol-1,
因此,本题正确答案是:-399.7。