��Ŀ����
6�� �����£���0.05mol/LNaOH��Һ�ֱ�ζ�10.00mLŨ�Ⱦ�Ϊ0.10mol/LCH3COOH�� Ka=l��10-5����ҺHCN��Ka=5��10-10����Һ�����õζ�������ͼ������˵����ȷ���ǣ�������
�����£���0.05mol/LNaOH��Һ�ֱ�ζ�10.00mLŨ�Ⱦ�Ϊ0.10mol/LCH3COOH�� Ka=l��10-5����ҺHCN��Ka=5��10-10����Һ�����õζ�������ͼ������˵����ȷ���ǣ�������| A�� | ����������ζ�����ѡ�ü�����ָʾ�� | |
| B�� | �۴���Һ�У�c��Na+��=c��CH3COO-����c��CH3COOH�� | |
| C�� | ��Һ��ˮ�ĵ���̶ȣ��ڣ��ۣ��� | |
| D�� | ��ٺ͵����Һ��Ϻ�c��CH3COO-��-c��CN-��=c��HCN��-c��CH3COOH�� |
���� A�����ȵı�ɫ��Χ��pH=3.1��4.4֮�䣬����ͼ�������0.10mol/L��HCN��pH���ڼ��ȵı�ɫ��Χ�ڣ�
B���۴���Һ��pH=7����Һ�����ԣ���Һ��c��H+��=c��OH-�������ݵ���غ�����жϣ�
C���ڴ���ҺpH��7����Һ�����ԣ��������Ϊ��������ˮ�ĵ��룬�۴���ҺpH=7����ʱ����CH3COONa��������CH3COOH���ܴ�Ϊ�ζ��յ㣬��Һ��ǡ������CH3COONa��CH3COONaˮ��ٽ�ˮ�ĵ��룬�ݴ˷�����
D����ٵ���Һ�д���CH3COONa��CH3COOH����ڵ���Һ�д���NaCN��HCN��������Һ�������غ㣬�����ͼ������жϣ�
��� �⣺A�����ȵı�ɫ��Χ��pH=3.1��4.4֮�䣬����ͼ�������0.10mol/L��HCN��pH���ڼ��ȵı�ɫ��Χ�ڣ���˵ζ�HCNʱ������ѡ�ü�����Ϊָʾ������A����
B���۴���Һ��pH=7����Һ�����ԣ���Һ��c��H+��=c��OH-�����������ζ��Ĵ�����Һ�����ݵ���غ㣬c��Na+��+c��H+��=c��OH-��+c��CH3COO-��������c��H+��=c��OH-������c��Na+��=c��CH3COO-������ʱ�μ�NaOH���������20mL�������Һ�л����ڼ�������CH3COOH����Ũ�ȹ�ϵӦΪc��Na+��=c��CH3COO-����c��CH3COOH������B����
C���ڴ���ҺpH��7����Һ�����ԣ��������Ϊ��������ˮ�ĵ��룬�۴���ҺpH=7����ʱ����CH3COONa��������CH3COOH���ܴ�Ϊ�ζ��յ㣬��Һ��ǡ������CH3COONa��CH3COONaˮ��ٽ�ˮ�ĵ��룬�����Һ��ˮ�ĵ���̶ȣ��ڣ��ۣ��ܣ���C����
D����ٵ���Һ�д���CH3COONa��CH3COOH�����������غ㣬c��CH3COO-��+c��CH3COOH��=0.05mol/L�������Һ�д���NaCN��HCN�����������غ㣬c��HCN��+c��CN-��=0.05mol/L������Һ��ϣ�����c��CH3COO-��+c��CH3COOH��=c��HCN��+c��CN-������c��CH3COO-��-c��CN-��=c��HCN��-c��CH3COOH������D��ȷ��
��ѡD��
���� ���⿼��������ʵĵ���ƽ�⣬�ζ�ԭ����ָʾ����ѡ������Ũ�ȴ�С�ıȽϣ����ͼ����������ΰ����غ�˼���ǽ���Ĺؼ��������Ѷ��еȣ�

| A�� | �����£�22.4LNH3��������ȫ��Ӧ����NOʱ��ת�Ƶĵ�����С��5NA | |
| B�� | ��1mol�ؼ���-CD3���к��еĵ�����Ϊ9NA | |
| C�� | �ڵ��ʳ��ˮ��ʵ���У���������Һ�����Ϊ1L����������������ķ�����Ϊ0.5NA��������Һ��pHΪ12 | |
| D�� | һ��������3mol SO2��1 molO2 ������ܱ������г�ַ�Ӧ��ķ�������Ϊ3NA |
| A�� | He | B�� |  | C�� | 1s2 | D�� | 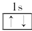 |
| A�� | ��SO2ͨ����ˮ��֤��SO2����Ư���� | |
| B�� | �������ſ������ռ�ͭ����ϡ���ᷴӦ������NO | |
| C�� | ����ˮ�����Һ©�����������Ҵ������ã��ɽ�����ȡ���Ҵ��� | |
| D�� | ����ϩͨ��KMnO4������Һ֤����ϩ���л�ԭ�� |
| A�� | ������ʫ�䡰ǧ�����������࣬������ɳʼ���𡱣��������ȶ�����ͨ�����������õ� | |
| B�� | ����ʯʫ�䡰��������һ�����������ů�����ա��������ȼ���漰������ԭ��Ӧ | |
| C�� | ���ʫ�䡰������¯�����̣�ң���ٲ���ǰ�����������̡�ָ����¯���е����������� | |
| D�� | ��ֲʫ�䡰��ȼ��ݽ�����ڸ������������ȼ��ݽ���������仯��Ҫ�ǻ�ѧ��ת��Ϊ���� |
| A�� | 500mL��Һ������K+��SO42-����Ϊ0.1NA | |
| B�� | 500mL��Һ�к���0.1NA��K+���� | |
| C�� | 1L��Һ��K+����Ũ����0.2mol/L | |
| D�� | 2L��Һ��SO42-����Ũ����0.2mol/L |
 ����ͭ���仯�������ճ����������������Ź㷺��Ӧ�ã��ش��������⣺
����ͭ���仯�������ճ����������������Ź㷺��Ӧ�ã��ش��������⣺ ��֪����FeCl2��Һ�еμ�ϡ���ᣬ��Һ��ɫ��dz��ɫ��Ϊ�ػ�ɫ��ijͬѧ�ô˷�Ӧ�������ͼ��ʾԭ��أ�
��֪����FeCl2��Һ�еμ�ϡ���ᣬ��Һ��ɫ��dz��ɫ��Ϊ�ػ�ɫ��ijͬѧ�ô˷�Ӧ�������ͼ��ʾԭ��أ�