��Ŀ����
������ԭ�ζ�ʵ��ͬ�к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.001 mol��L-1KMnO4������Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ�����ӷ���ʽ�ǣ�2![]() +5
+5![]() +H+====2Mn2++5
+H+====2Mn2++5![]() +3H2O
+3H2O
��ջش����⣺
��1���õζ�ʵ�����������������е�______________������ţ���
A.��ʽ�ζ��ܣ�50 mL�� B.��ʽ�ζ��ܣ�50 mL��
C.��Ͳ��10 mL�� D.��ƿ
E.����̨ F.�ζ��ܼ�
G.�ձ� H.��ֽ
I.��ͷ�ι� J.©��
��2������___________����д���ᡱ�����ʽ�ζ���ʢװ���������Һ���Է���ԭ��
____________________________��
��3��ѡ����ָʾ����˵������________________________________��
��4���ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����b-a�� mL����ʵ������KMnO4��Һ���_________���ࡢ�٣������ݣ�b-a�� mL���㣬�õ��Ĵ���Ũ�ȣ���ʵ��Ũ��________����С����
��1��A��B��D��E��F��G��H ��2���� KMnO4�ܰ���������ʹ���ϻ� ��3������ָʾ������ΪKMnO4����ԭ����ԭ��Mn2+����ɫ��ȥ�����Բ���Ҫָʾ�� ��4���� С
������������ԭ�ζ�ԭ������������к͵ζ�ԭ��������KMnO4��Һ����ɫ����ǿ�����ԣ���Ӧ����Һ��ɫ��ȥ�����ѡ��ζ���ʱ�������ü�ʽ�ζ���ʢװKMnO4��Һ������KMnO4��Һ��ɫ�ı仯���Ϳ����ж��Ƿ�Ӧ��ȫ����˲���Ҫָʾ����

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�ʵ����
��1���ռ��ڱ�����̻Ჿ�ֱ��ʣ�������ҪΪNa2CO3����
ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ����ȡ���ƺõ��ռ���Һ10.00mL����ƿ�У��ֱ�����ƿ�и�����1��2�η�ָ̪ʾ��������֪����̪��ɫʱ����ʱֻ��NaOH��HCl��Ӧ��Na2CO3��û����HCl��Ӧ����Ũ��Ϊ0.20mol��L-1�������Һ���еζ���������ݼ�¼���£�
|
ʵ���� |
V���ռ���Һ��/mL |
V�����ᣩ/mL |
|
|
��ʼ���� |
ĩβ���� |
||
|
1 |
10.00 |
0.50 |
21.52 |
|
2 |
10.00 |
1.00 |
21.98 |
|
3 |
10.00 |
0.20 |
24.80 |
�Իش�
�ٵζ�ʱ���ֵ���ȷ������______________________________________��
���жϵ���ζ��յ��ʵ��������__________________________________________�����ݱ������ݣ�������ռ���Ʒ�к�NaOH����������Ϊ______ _����
�����в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���__________��
A����ƿ������ˮϴ��δ�ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ζ�����������ƿʱ��������������Һ����
D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
��2��������ԭ�ζ�ʵ��ͬ�к͵ζ����ơ�������������Ҫ��������������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȡ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ�˹��������Ũ�ȡ���֪��2KMnO4��5H2O2��6H2SO4===2MnSO4��8H2O��5O2��������д���пհף�
���� (���ʽ����ʽ��)�ζ�����ȡ����������Һ25.00 mL����ƿ�У�������������
�ڵζ�ʱ����������ر���Һע����ʽ�ζ����У�������ر���Һ����ʽ�ζ���ԭ��Ϊ �����ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й�����������ʵ���Ũ��Ϊ ��
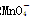 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5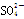 ��3H2O����ջش����⣺
��3H2O����ջش����⣺