��Ŀ����
����Ŀ��ij�л���������ת����ϵ��

��1��д����ӦA��B�ķ�Ӧ������____________��C�й����ŵĽṹ��ʽ��______________��
��2��д����ӦB��C�Ļ�ѧ����ʽ�ͷ�Ӧ���ͣ�_______________����Ӧ����Ϊ____________��
��3�� ��Ũ������������������ɺ���������Ԫ���ṹ���л�����л���ṹ��ʽΪ��____________��
��Ũ������������������ɺ���������Ԫ���ṹ���л�����л���ṹ��ʽΪ��____________��
��4��д����������������C��ͬ���칹��Ľṹ��ʽ______________��_____________��
�����ڷ����廯���������������ȡ����
����̼��������Һ��Ӧ�ų�������̼����
�۱����ϵ�һ�ȴ���������
��5��д���� ��CH3CH2Cl��Ϊԭ�Ϻϳ���Ҫ�Ļ�����Ʒ
��CH3CH2Cl��Ϊԭ�Ϻϳ���Ҫ�Ļ�����Ʒ![]() ��·������ͼ�����Լ���ѡ��______��
��·������ͼ�����Լ���ѡ��______��
���𰸡� ��������ˮ��Һ������ -OH��-CHO 2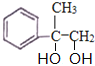 + O2
+ O2![]() 2
2 + 2H2O ������Ӧ
+ 2H2O ������Ӧ 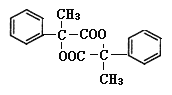
![]()
![]()
�������� ����ˮ�ӳ�����A��AΪ
����ˮ�ӳ�����A��AΪ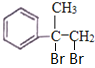 ��B���������ɵ�C�ܹ�����������Ӧ��˵��CΪȩ����BΪ�������Aˮ������B��BΪ
��B���������ɵ�C�ܹ�����������Ӧ��˵��CΪȩ����BΪ�������Aˮ������B��BΪ ���������ղ���Ľṹ��֪��CΪ
���������ղ���Ľṹ��֪��CΪ ��
��
��1����ӦA��BΪ±������ˮ�ⷴӦ����Ӧ����Ϊ��������ˮ��Һ�����ȣ�CΪ �����еĹ����ŵĽṹ��ʽΪ-OH��-CHO���ʴ�Ϊ����������ˮ��Һ�����ȣ� -OH��-CHO��
�����еĹ����ŵĽṹ��ʽΪ-OH��-CHO���ʴ�Ϊ����������ˮ��Һ�����ȣ� -OH��-CHO��
��2����ӦB��CΪ�ǻ��Ĵ���������ȩ������Ӧ�Ļ�ѧ����ʽΪ2 + O2
+ O2![]() 2
2 + 2H2O���ʴ�Ϊ��2
+ 2H2O���ʴ�Ϊ��2 + O2
+ O2![]() 2
2 + 2H2O��������Ӧ��
+ 2H2O��������Ӧ��
��3��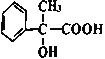 ��Ũ������������������ɺ���������Ԫ���ṹ���л��Ϊ2����
��Ũ������������������ɺ���������Ԫ���ṹ���л��Ϊ2����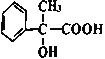 �������Ӽ��������Ӧ������Ľṹ��ʽΪ
�������Ӽ��������Ӧ������Ľṹ��ʽΪ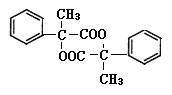 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
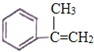
��4��CΪ �������ڷ����廯���������������ȡ����������̼��������Һ��Ӧ�ų�������̼���壬˵���ṹ�к����Ȼ����۱����ϵ�һ�ȴ��������֣���������������C��ͬ���칹����
�������ڷ����廯���������������ȡ����������̼��������Һ��Ӧ�ų�������̼���壬˵���ṹ�к����Ȼ����۱����ϵ�һ�ȴ��������֣���������������C��ͬ���칹����![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
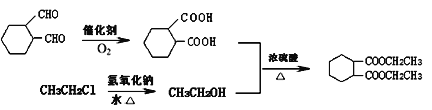
��5����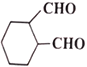 ��CH3CH2ClΪԭ�Ϻϳ�
��CH3CH2ClΪԭ�Ϻϳ�![]() ����Ҫ���Ⱥϳ�
����Ҫ���Ⱥϳ�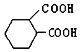 ���Ҵ����ϳ�
���Ҵ����ϳ� ������
������ �����õ����ϳ��Ҵ�������CH3CH2Clˮ��õ�����˺ϳ�·������Ϊ
�����õ����ϳ��Ҵ�������CH3CH2Clˮ��õ�����˺ϳ�·������Ϊ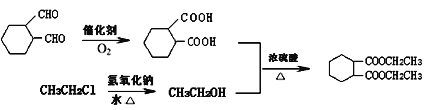 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�





