ƒøƒ⁄»ð
°æƒø°øŒ™¡ÀºÏ≤‚ Ï»‚÷–NaNO2∫¨¡ø£¨ƒ≥–À»§–°◊È¥”1 000 g ∏Ù“π Ï»‚÷–÷»°NaNO3∫ÕNaNO2∫Û≈‰≥…»Ð“∫£¨”√0.005 00 mol°§L£≠1µƒ∏þ√ÃÀ·ºÿ(À·–‘)»Ð“∫µŒ∂®°£∆Ω––≤‚∂®»˝¥Œ£¨«Û≥ˆ√ø¥ŒNaNO2∫¨¡ø£¨»°∆‰∆Ωæ˘÷µ°£(“—÷™£∫2MnO![]() £´5NO
£´5NO![]() £´6H£´===2Mn2£´£´5NO
£´6H£´===2Mn2£´£´5NO![]() £´3H2O)
£´3H2O)
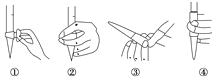
£®1£©µŒ∂®«∞≈≈∆¯≈ð ±£¨”¶—°‘ÒÕº÷–µƒ________(ÃÓ–Ú∫≈)°£µŒ∂® ±£¨∏þ√ÃÀ·ºÿ»Ð“∫ ¢∑≈‘⁄___________________°£
£®2£©µŒ∂®÷’µ„µƒ≈–∂œ“¿æðŒ™__________________________________°£
£®3£©œ¬¡–≤Ÿ◊˜ª·µº÷¬—˘∆∑∫¨¡ø≤‚∂®÷µ∆´∏þµƒ «__________________(ÃÓ–Ú∫≈)°£
a£Æ◊∂–Œ∆ø”√’Ù¡ÛÀÆœ¥∫ÛŒ¥”√¥˝≤‚“∫»Ûœ¥
b£ÆÀ· ΩµŒ∂®πД√’Ù¡ÛÀÆœ¥∫ÛŒ¥”√±Í◊º“∫»Ûœ¥
c£ÆµŒ∂®π˝≥Ã÷–’Òµ¥◊∂–Œ∆ø ±£¨”–…Ÿ¡ø¥˝≤‚»Ð“∫Ω¶≥ˆ
d£ÆµŒ∂®«∞∆Ω ”∂¡ ˝£¨µŒ∂®Ω· ¯—ˆ ”∂¡ ˝
£®4£©ƒ≥¥ŒµŒ∂®π˝≥Ã÷–£¨œ˚∫ƒ∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝Œ™16.00 mL°£‘Ú¥À¥Œ«Ûµ√µƒNaNO2µƒ∫¨¡øŒ™________mg°§kg£≠1°£
°æ¥∞∏°ø ¢⁄ À· ΩµŒ∂®πÐ µ±µŒ»Î◊Ó∫Û“ªµŒ(ªÚ∞εŒ)∏þ√ÃÀ·ºÿ»Ð“∫ ±£¨»Ð“∫—’…´”…ŒÞ…´±‰Œ™◊œ∫Ï…´£¨«“∞Î∑÷÷”ƒ⁄≤ªÕ …´£¨º¥Œ™µŒ∂®÷’µ„ bd 13.8
°æΩ‚Œˆ°ø ‘Â∑÷Œˆ£∫£®1£©∏þ√ÃÀ·ºÿ»Ð“∫∏Ø ¥œΩ∫£¨”¶ ¢‘⁄À· ΩµŒ∂®πÐ÷–£¨Œ™æ´»∑øÿ÷∆≈≈≥ˆ∆¯≈ð£¨◊Û ÷Œ’◊°À· ΩµŒ∂®πЪӻ˚≈≈∆¯£ª£®2£©µŒ»Îµƒ∏þ√ÃÀ·ºÿ»Ð“∫≤ª‘ŸÕ …´ ±¥ÔµΩµŒ∂®÷’µ„£ª£®3£©∏˘æð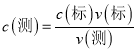 ∑÷ŒˆŒÛ≤Ó£ª£®4£©∏˘æð2MnO
∑÷ŒˆŒÛ≤Ó£ª£®4£©∏˘æð2MnO![]() £´5NO
£´5NO![]() £´6H£´===2Mn2£´£´5NO
£´6H£´===2Mn2£´£´5NO![]() £´3H2Oº∆À„œ˚∫ƒ∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝Œ™16.00 mL ±£¨NaNO2µƒ÷ ¡ø°£
£´3H2Oº∆À„œ˚∫ƒ∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝Œ™16.00 mL ±£¨NaNO2µƒ÷ ¡ø°£
Ω‚Œˆ£∫£®1£©∏þ√ÃÀ·ºÿ»Ð“∫ƒÐ∏Ø ¥œΩ∫£¨À˘“‘∏þ√ÃÀ·ºÿ»Ð“∫ ¢‘⁄À· ΩµŒ∂®πÐ÷–£¨Œ™æ´»∑øÿ÷∆≈≈≥ˆ∆¯≈ð£¨◊Û ÷Œ’◊°À· ΩµŒ∂®πЪӻ˚≈≈∆¯£¨À˘“‘”¶—°‘ÒÕº÷–µƒ¢⁄£ª£®2£©µŒ∂®÷’µ„ ±£¨∏þ√ÃÀ·ºÿ»Ð“∫±ªÕÍ»´œ˚∫ƒ£¨µ±µŒ»Î◊Ó∫Û“ªµŒ(ªÚ∞εŒ)∏þ√ÃÀ·ºÿ»Ð“∫ ±£¨»Ð“∫—’…´”…ŒÞ…´±‰Œ™◊œ∫Ï…´£¨«“∞Î∑÷÷”ƒ⁄≤ªÕ …´£¨º¥Œ™µŒ∂®÷’µ„£ª£®3£©a£Æ◊∂–Œ∆ø”√’Ù¡ÛÀÆœ¥∫ÛŒ¥”√¥˝≤‚“∫»Ûœ¥£¨≤ª”∞œÏΩ·π˚£¨π a¥ÌŒÛ£ªb£ÆÀ· ΩµŒ∂®πД√’Ù¡ÛÀÆœ¥∫ÛŒ¥”√±Í◊º“∫»Ûœ¥£¨±Í◊º“∫≈®∂»±‰–°£¨œ˚∫ƒ∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝∆´¥Û£¨≤‚∂®÷µ∆´∏þ£¨π b’˝»∑£ªc£ÆµŒ∂®π˝≥Ã÷–’Òµ¥◊∂–Œ∆ø ±£¨”–…Ÿ¡ø¥˝≤‚»Ð“∫Ω¶≥ˆ£¨œ˚∫ƒ∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝∆´–°£¨≤‚∂®÷µ∆´µÕ£¨π c¥ÌŒÛ£ªd£ÆµŒ∂®«∞∆Ω ”∂¡ ˝£¨µŒ∂®Ω· ¯—ˆ ”∂¡ ˝£¨∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝∆´¥Û£¨≤‚∂®÷µ∆´∏þ£¨π d’˝»∑£ª£®4£©œ˚∫ƒ∏þ√ÃÀ·ºÿ»Ð“∫µƒÃª˝Œ™16.00 mL£¨n(KMnO4)=0.016L°¡0.005 00 mol°§L£≠1=0.00008mol£¨∏˘æð2MnO![]() £´5NO
£´5NO![]() £´6H£´===2Mn2£´£´5NO
£´6H£´===2Mn2£´£´5NO![]() £´3H2O£¨n(NaNO2)= 0.00008mol°¬2°¡5=0. 0002mol£¨ NaNO2µƒ÷ ¡ø «0.0138g=13.8mg£¨NaNO2µƒ∫¨¡øŒ™13.8mg°§kg£≠1°£
£´3H2O£¨n(NaNO2)= 0.00008mol°¬2°¡5=0. 0002mol£¨ NaNO2µƒ÷ ¡ø «0.0138g=13.8mg£¨NaNO2µƒ∫¨¡øŒ™13.8mg°§kg£≠1°£

°æƒø°øœ¬¡–≥˝‘”∑Ω∑®—°”√¥ÌŒÛµƒ «
ŒÔ÷ (¿®∫≈ƒ⁄Œ™‘”÷ ) | ≥˝‘”∑Ω∑® | |
A | œıª˘±Ω(±Ω) | ’Ù¡Û |
B | ““œ©(SO2) | NaOH»Ð“∫£¨œ¥∆¯ |
C | º∫ÕÈ(º∫œ©) | ‰ÂÀÆ£¨∑÷“∫ |
D | µÌ∑€»Ð“∫(NaCl) | …¯Œˆ |
A.AB.BC.CD.D
°æƒø°øº◊°¢““°¢±˚°¢∂°°¢ŒÏŒÂ÷÷ŒÔ÷ ÷–£¨º◊°¢““°¢±˚÷–æ˘∫¨”–ƒ≥÷÷œýÕ¨µƒ‘™Àÿ£¨À¸√«÷ƺ‰æþ”–»ÁÕºÀ˘ æ◊™ªØπÿœµ(∑¥”¶Ãıº˛º∞≤ø∑÷≤˙ŒÔ“—¬‘»•)°£œ¬¡–”–πÿŒÔ÷ µƒÕ∆∂œ≤ª’˝»∑µƒ «(°°°°)

—°œÓ | ºŸ…Ë | Ω·¬€ |
A | º◊Œ™Al(OH)3 | ∂°ø…ƒÐ «—ŒÀ· |
B | º◊Œ™Na2CO3»Ð“∫ | ŒÏø…ƒÐ «CO2 |
C | º◊Œ™Fe | ∂°ø…ƒÐ «—ŒÀ· |
D | º◊Œ™N2 | ŒÏø…ƒÐ «—ı∆¯ |
A. A B. B C. C D. D