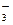��Ŀ����
���й�ϵʽ����ȷ����
c(CO32��)��2c(OH��) c(HCO3�� )��3c(H2CO3)��2c(H��)
c(HCO3�� )��3c(H2CO3)��2c(H��)
| A������������ʵ���Ũ�ȵ�NaClO(aq)��NaCl(aq)������������С��Nǰ��N�� |
B��0.1 mol��L��1Na2S��Һ�У�2c(Na��) c(S2��)��c(HS��)��c(H2S) c(S2��)��c(HS��)��c(H2S) |
| C�������½������ơ���������Һ��Ϻ���Һ�����ԣ����Ϻ���Һ�У� c(Na��)��c(Cl��)��c(CH3COOH) |
| D��0.1 mol��L��1Na2CO3��Һ��0.1 mol��L��1NaHCO3��Һ�������� |
 c(HCO3�� )��3c(H2CO3)��2c(H��)
c(HCO3�� )��3c(H2CO3)��2c(H��)D
ѡ��A��NaClO(aq)��NaCl(aq)�е���غ�ʽ�ֱ�Ϊc(Na��)��c(H��)
 c(ClO��)��c(OH��)��c(Na��)��c(H��)
c(ClO��)��c(OH��)��c(Na��)��c(H��) c(Cl��)��c(OH��)������NaClOˮ���Լ��ԣ������c(H��)NaClO��c(H��)NaCl������NNaClO��NNaCl��A����ѡ��B�е������غ�ʽӦΪ
c(Cl��)��c(OH��)������NaClOˮ���Լ��ԣ������c(H��)NaClO��c(H��)NaCl������NNaClO��NNaCl��A����ѡ��B�е������غ�ʽӦΪ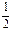 c(Na��)
c(Na��) c(S2��)��c(HS��)��c(H2S)��ѡ��C����������������д�������Һ�ĵ���غ�ʽ��c(H��)��c(Na��)
c(S2��)��c(HS��)��c(H2S)��ѡ��C����������������д�������Һ�ĵ���غ�ʽ��c(H��)��c(Na��) c(OH��)�� c(Cl��)�� c(CH3COO��)����������Һ�����Լ�c(H��)��c(OH��)����˻����Һ��Ӧ��c(Na��)
c(OH��)�� c(Cl��)�� c(CH3COO��)����������Һ�����Լ�c(H��)��c(OH��)����˻����Һ��Ӧ��c(Na��) c(Cl��)��c(CH3COO��)�����������غ�ɵ�c(Na��)
c(Cl��)��c(CH3COO��)�����������غ�ɵ�c(Na��) c(CH3COOH)��c(CH3COO��)��������c(Na��)��c(Cl��)
c(CH3COOH)��c(CH3COO��)��������c(Na��)��c(Cl��) c(CH3COOH)��C����
c(CH3COOH)��C����
��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
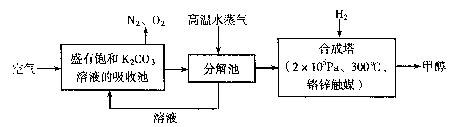
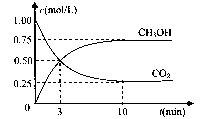
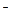 ����c��H+��+ c��CH3COOH��
����c��H+��+ c��CH3COOH�� ��Cl-��NH
��Cl-��NH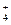 B��Ba2����Mg2����NO
B��Ba2����Mg2����NO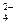 ��Cl-��K����NH
��Cl-��K����NH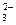 ��K����AlO
��K����AlO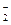 ��Na��
��Na�� ���������ܴ����������
���������ܴ����������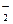 ��ClO��
��ClO��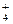 ��Cl����NO
��Cl����NO