��Ŀ����
����Ŀ��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

(1)��д��Ӳ�ʲ������з����Ļ�ѧ��Ӧ����ʽ______________________��
(2)�״���Ҫ����ˮԡ���ȣ�Ŀ����_______________________________��
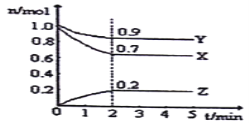
(3)��Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������___________������ƿ���ռ������������Ҫ�ɷ���__________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л���_____��Ҫ��ȥ�����ʣ������ڻ��Һ�м���_______ (��д��ĸ)��Ȼ����ͨ�����ɳ�ȥ��
A���Ȼ�����Һ B���� C��̼��������Һ D�����Ȼ�̼
���𰸡�2Cu+O2![]() 2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO![]() CH3CHO+Cu+H2O����2CH3CH2OH+O2
CH3CHO+Cu+H2O����2CH3CH2OH+O2![]() 2CH3CHO+2H2O �����Ҵ�����(������Ҵ��Ļӷ� ) ��ȩ���Ҵ���ˮ ����(��N2) ���� C
2CH3CHO+2H2O �����Ҵ�����(������Ҵ��Ļӷ� ) ��ȩ���Ҵ���ˮ ����(��N2) ���� C
��������
(1)�Ҵ���ͭ������������������������ȩ��
(2)�����Ҵ����������ʣ��Ҵ��ӷ����Ҵ��Ƿ�Ӧ�Ӧת�����Ҵ��������뵽Ӳ���Թ��ڲ��뷴Ӧ���ʼ״���Ҫ����ˮԡ���ȣ�
(3)�������ʵķе�ߵͲ�ͬ��ȷ����õ����ʣ���Ͽ����ijɷ��Լ������ķ�Ӧȷ��ʣ�������ɷ֣�
(4)��ʹ��ɫʯ����ֽ�������ᣬ̼�����ƿ��Ժ����ᷴӦ��
(1)�Ҵ���ͭ������������������������ȩ������ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��ˣ�������ȷ���ǣ�2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(2)�����Ҵ����������ʣ��Ҵ��ӷ����Ҵ��Ƿ�Ӧ�Ӧת�����Ҵ��������뵽Ӳ���Թ��ڲ��뷴Ӧ���ʼ״���Ҫ����ˮԡ���ȣ�
��ˣ�������ȷ���ǣ������Ҵ�����(������Ҵ��Ļӷ� )��
(3)�Ҵ��Ĵ�����ʵ���е����ʣ��Ҵ�����ȩ��ˮ�ķе�ߵͲ�ͬ�����Թ�a�����ռ���Щ��ͬ�����ʣ������ijɷ���Ҫ�ǵ����������������μӷ�Ӧ��ʣ�����Ҫ�ǵ�����
��ˣ�������ȷ���ǣ���ȩ���Ҵ���ˮ��������
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л��������ᣬ�ĸ�ѡ����У�ֻ��̼�����ƿ��Ժ����ᷴӦ�����������ơ�ˮ�Ͷ�����̼��ʵ�����ֻ������ʵķ���������
��ˣ�������ȷ���ǣ����C��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�����Ŀ�����±���Ԫ�����ڱ���һ���֣��û�ѧ����ش��������⣺
�� ���ڡ� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | �� | ||
4 |
(1)�뻭��Ԫ�آ������ӵĽṹʾ��ͼ��_________��
(2)�ۢݢ��ԭ�Ӱ뾶��С�����˳��Ϊ__________(��Ԫ�ط���)��
(3)�ݺ͢ߵ�����������Ӧˮ����ļ���ǿ��Ϊ______��______(�ѧʽ)��
(4)�ۢ�����Ԫ�ص�ԭ�Ӱ�1��1��ɵij���������ĵ���ʽΪ___________��
(5)�õ���ʽ��ʾ�͢��γɵĻ�����Ĺ��̣�________________��
��������ͼװ�ÿ���֤ͬ����Ԫ�طǽ����Եı仯����
(1)��Ҫ֤���ǽ����ԣ�Cl>I����A�м�Ũ���ᣬB�м�KMnO4����KMnO4��Ũ���᳣���·�Ӧ������������C�мӵ��۵⻯�ػ����Һ���۲쵽C����Һ������________������֤������д��һ����ȡ�����Ļ�ѧ��Ӧ����ʽ��_______���ӻ��������ĽǶȿ��ǣ���װ��ȱ��β������װ�ã�����___________��Һ����β����
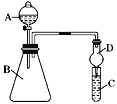
(2)��Ҫ֤���ǽ����ԣ�C>Si������A�м����ᡢB�м�CaCO3��C�м�Na2SiO3��Һ���۲쵽C����Һ__________��������֤�������е�ͬѧ��Ϊ������лӷ��ԣ��ɽ���C�и���ʵ�飬Ӧ����װ�ü�����װ��_______��Һ��ϴ��ƿ��