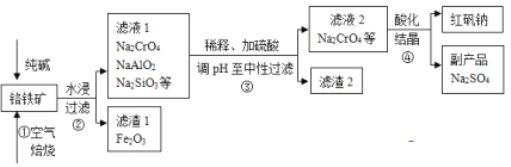
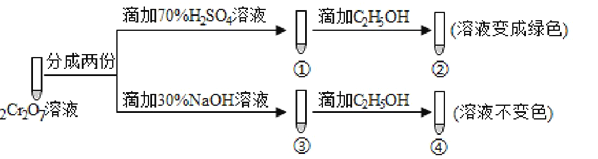
��Ŀ����
����Ŀ��A��B��C��D��E Ϊ����������Ԫ�أ���ԭ��������������B��C��D��E λ��ͬһ���ڣ� A �ļ���̬�⻯����������������1 mol B �������������ᷴӦ���������ڱ�״���µ����Ϊ33.6 L��D ԭ������������������������֮��Ϊ 3��8��C ��ԭ���������� A ��������
(1)A��B �����Ӱ뾶�ɴ�С��˳��Ϊ______________ (�����ӷ���) ��
(2)C ��Ԫ�����ڱ��е�λ����_______________ ��
(3)A �ļ���̬�⻯��Ӵ��� E �ļ���̬�⻯��ʱ�ɹ۲쵽��������_____________��
(4) D �� E ���γ�ԭ�Ӹ�����Ϊ 1��2 �Ļ���������ʽΪ________��
(5)C��D��E �����̬�⻯���ȶ����ɸߵ��͵�˳����___________ (�û�ѧʽ���� )��
(6)B �� D �γɵĻ�������ˮѸ��ˮ�⣬д���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
(7)�� E ������ NaOH ��Һ��Ӧ���� NaE��NaEO �� NaEO3���� 30mL 2mol/LNaOH ��_____mol E ����ǡ����ȫ��Ӧ(���� E ������ˮ�ķ�Ӧ�������ˮ�ⷴӦ)��
���𰸡�r(N3)��r(Al3+) �� N3��Al3+ �������� �ڢ�A �� �������� ![]() HCl��H2S��SiH4 Al2S3��6H2O =2Al(OH)3����3H2S�� 0.03
HCl��H2S��SiH4 Al2S3��6H2O =2Al(OH)3����3H2S�� 0.03
��������
A��B��C��D��EΪ�����ڵ�����Ԫ�أ���ԭ��������������B��C��D��Eλ��ͬһ���ڣ�A �ļ���̬�⻯������������������AΪNԪ�أ�1 mol B �������������ᷴӦ���������ڱ�״���µ����Ϊ 33.6 L����BΪAlԪ�أ�D ԭ������������������������֮��Ϊ 3��8����Dλ�ڵ������ڣ���DΪSԪ�أ�C ��ԭ��������A����������CΪSiԪ�أ�EԪ��Ϊ����������Ԫ����ԭ����������D����EΪClԪ�أ�
�������Ϸ�����֪��A��B��C��D��E�ֱ�ΪN��Al��Si��S��Cl��
��1��AΪNԪ����BΪAlԪ�أ���������Ų���ͬ���˵����Խ��뾶ԽС�������Ӱ뾶N3��Al3+��
��ˣ�������ȷ��Ϊ��N3��Al3+��
��2��CΪSiԪ���� ��Ԫ�����ڱ��е�λ���ǵ������� �ڢ�A �塣
��ˣ�������ȷ��Ϊ���������� �ڢ�A �壻
��3��A ���⻯���ǰ�����E���⻯����HCl��������ӽ��а������ɣ���ɷ�Ϊ�Ȼ�粒�����
��ˣ�������ȷ��Ϊ���������̣�
��4��D��SԪ�ء�E��ClԪ�أ�D��E���γ�ԭ�Ӹ�����Ϊ1��2�Ļ�����ΪSCl2��ÿ��Clԭ�Ӻ�Sԭ���γ�һ�Թ��õ��Ӷԣ������ʽΪ![]() ��
��
��ˣ�������ȷ��Ϊ��![]() ��
��
(5) CΪSiԪ�ء�DΪSԪ�ء�EΪClԪ�أ��ǽ�����Cl��S��Si�������⻯����ȶ���HCl��H2S��SiH4��
��ˣ�������ȷ��Ϊ��HCl��H2S��SiH4��
(6) BΪAlԪ�ء�DΪSԪ�أ�B��D�γɵĻ�����ΪΪAl2S3����ˮѸ��ˮ�⣬��Ӧ�Ļ�ѧ����ʽΪAl2S3��6H2O =2Al(OH)3����3H2S����
��ˣ�������ȷ��Ϊ��Al2S3��6H2O =2Al(OH)3����3H2S����
(7)EΪClԪ������Cl2�� NaOH ��Һ��Ӧ���� NaCl��NaClO �� NaClO3������ԭ���غ�
n(Cl2)=![]() n(Cl)=
n(Cl)=![]() n(Na)=
n(Na)=![]() n(NaOH)=0.030L�� 2mol/L��
n(NaOH)=0.030L�� 2mol/L��![]() =0.03mol��
=0.03mol��
��ˣ�������ȷ��Ϊ��0.03��

 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�����Ŀ���±�Ϊԭ��������������Ķ�����Ԫ��A~F�ĵ�һ��������������ݣ����еĽ���Ԫ����
������I��ev�� | A | B | C | D | E | F |
I1 | 5.2 | 7.6 | 6.0 | 11.3 | 13.6 | 14.5 |
I2 | 49.3 | 15.0 | 18.8 | 24.4 | 35.1 | 29.6 |
I3 | 71.6 | 80.1 | 28.4 | 47.9 | 54.9 | 47.4 |
I4 | 98.9 | 109.2 | 112.0 | 64.5 | 77.4 | 77.5 |
I5 | 138.3 | 141.3 | 153.7 | 392.1 | 113.9 | 97.9 |
A.A B CB.B C DC.C D ED.D E F