��Ŀ����
(1)ij�¶�(t��)ʱ��ˮ�����ӻ�ΪKw��1��10��13������¶ȡ���25�棨��������������������ڴ��¶��£�ij��Һ����ˮ���������H��Ũ��Ϊ1��10��10mol/L�������Һ��pH����Ϊ�� ������ ��
���¶�Ϊ25��ʱ�����ΪVa��pH��a��H2SO4��Һ�����ΪVb��pH��b��NaOH��Һ��ϣ�ǡ���к�.��֪Va��Vb����a��0.5 b����a��ȡֵ��Χ������������������
��3���������¶�(t��)�£�pH��11�Ŀ�������Һm L��pH��1��ϡ������ҺnL���(�����Ϻ���Һ�����С�仯���Բ���)����ͨ��������д���²�ͬ���ʱ������Һ������ȣ����Ƚ���Һ�и����ӵ�Ũ�ȴ�С��
�������û��ҺΪ���ԣ���m��n����������������Һ�и������ӵ�Ũ���ɴ�С����˳��������������������������������������������
�������û��Һ��pH��2����m��n��������������Һ�и������ӵ�Ũ���ɴ�С����˳������������������������������������������������
���¶�Ϊ25��ʱ�����ΪVa��pH��a��H2SO4��Һ�����ΪVb��pH��b��NaOH��Һ��ϣ�ǡ���к�.��֪Va��Vb����a��0.5 b����a��ȡֵ��Χ������������������
��3���������¶�(t��)�£�pH��11�Ŀ�������Һm L��pH��1��ϡ������ҺnL���(�����Ϻ���Һ�����С�仯���Բ���)����ͨ��������д���²�ͬ���ʱ������Һ������ȣ����Ƚ���Һ�и����ӵ�Ũ�ȴ�С��
�������û��ҺΪ���ԣ���m��n����������������Һ�и������ӵ�Ũ���ɴ�С����˳��������������������������������������������
�������û��Һ��pH��2����m��n��������������Һ�и������ӵ�Ũ���ɴ�С����˳������������������������������������������������
(1)����10 3��ÿ��1�֣� (2)
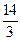 ��a��7��2�֣�
��a��7��2�֣�(3) ��10��1��2�֣���c(Na��)��c(SO)��c(H��)��c(OH��) ��ȫ�Ե�2�֣�
��9��2��2�֣���c(H��)��c(SO)��c(Na��)��c(OH��) ��ȫ�Ե�2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 4�� �� HSO4��
4�� �� HSO4�� H++SO42�����ش��������⣺
H++SO42�����ش��������⣺ ����ʽ��ʾ�� ��
����ʽ��ʾ�� ��
 (SO42��)+c(HSO4��)+c(H2SO4)="0.1" mol��L-1
(SO42��)+c(HSO4��)+c(H2SO4)="0.1" mol��L-1 NH3��+SO32- +2H2O?
NH3��+SO32- +2H2O? Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ ��
Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ �� 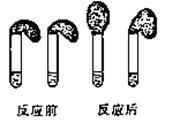
 l��Һ��100mL��
l��Һ��100mL��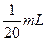 ���ֱ�ϡ����100mL��
���ֱ�ϡ����100mL�� ��L-1H2SO4 D��2mol��L-1HA
��L-1H2SO4 D��2mol��L-1HA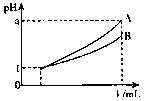
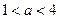 ����A��B��������
����A��B�������� H+ + HSO3- HSO3-
H+ + HSO3- HSO3-