��Ŀ����
����Ŀ�������ѱ���Ϊ��21���ͽ�����
(1)��ҵ�����ѿ�ʯ(��FeTiO3����FeO��Al2O3��SiO2������)�������������Ƶ�TiO2��
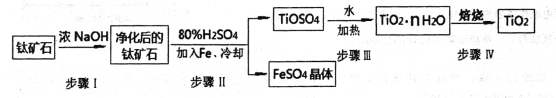
���У����������ӦΪ��2H2SO4+FeTiO3=TiOSO4+FeSO4+2H20
������I�����Ļ�ѧ����ʽ��______________________________��������FeSO4��������ˮ���������ɺ����õĹ�����_________________
�����������������Ti4+����Һ��ˮ���Ƶ�TiO2��nH2O,��÷�Ӧ�����ӷ���ʽΪ_________________________________________________
(2)������TiO2ͨ���������ַ����Ʊ������ѣ�
����һ����TiO2��������ʯī������������CaOΪ���Һ����̿�������۳أ����TiO2�Ƶ��ѣ������������ķ�Ӧ��______________________________________��
��������ͨ�����·�Ӧ�Ʊ�������
��TiO2(s)+2Cl2(g) ![]() TiCl4(g)+O2(g) ��H=+151 KJ/mol
TiCl4(g)+O2(g) ��H=+151 KJ/mol
��TiCl4+2Mg![]() 2MgCl2+Ti
2MgCl2+Ti
��ʵ�������У����ڷ�Ӧ�ٹ����м���̼����˳���Ƶ�TiCl4����ԭ����_________________________��______________________________________________��(������)
(3)����֪��C(s)+O2(g)=CO2(g) ��H=-394 KJ/mol,���ɹ���TiO2������C��Cl2��Ӧ��ȡ��̬TiCl4���Ȼ�ѧ����ʽΪ______________________________________________��
���𰸡�Al2O3+2NaOH=NaAlO2+H2O SiO2+2NaOH=Na2SiO3+H2O Fe2O3��Fe2(SO4)3 Ti4++(n+2)H2O ![]() TiO2��nH2O+4H+ TiO2+4e-=Ti+2O2- ̼������������Ӧ��С����Ũ��ʹƽ�������ƶ��������÷�Ӧ���ȣ����·�Ӧ˳�����У�ʹ���ɸ���TiCl4 TiO2(s) + 2Cl2��g��+ C��s��= TiCl4��g��+ CO2(g)��H=-243KJ/mol
TiO2��nH2O+4H+ TiO2+4e-=Ti+2O2- ̼������������Ӧ��С����Ũ��ʹƽ�������ƶ��������÷�Ӧ���ȣ����·�Ӧ˳�����У�ʹ���ɸ���TiCl4 TiO2(s) + 2Cl2��g��+ C��s��= TiCl4��g��+ CO2(g)��H=-243KJ/mol
��������
������Ҫ������ڹ�ҵ���ѷ��������ۡ�
��1���ٲ���IAl2O3��SiO2����Ũ����������Һ����Ӧ�Ļ�ѧ����ʽ��Al2O3+2NaOH=NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O����������FeSO4��������ˮ���������ɣ�������FeSO4����������������ΪFe2(SO4)3��ˮ����������������ȷֽ���������������ù�����Fe2O3��Fe2(SO4)3��
������������������Ti4+����Һ��ˮ���Ƶ�TiO2��nH2O����÷�Ӧ�����ӷ���ʽΪTi4++(n+2)H2O![]() TiO2��nH2O+4H+��
TiO2��nH2O+4H+��
��2������һ����TiO2��������ʯī������������CaOΪ���Һ����̿�������۳أ����TiO2�Ƶ��ѣ������������ķ�Ӧ��TiO2+4e-=Ti+2O2-��
����������ʵ�������У����ڷ�Ӧ�ٹ����м���̼����˳���Ƶ�TiCl4����ԭ����̼������������Ӧ��С����Ũ��ʹƽ�������ƶ��������÷�Ӧ���ȣ���ʹ��Ӧ˳�����У����ɸ���TiCl4����
��3�����Ȼ�ѧ����ʽ��C(s)+O2(g)=CO2(g) ��H=-394 KJ/mol���â۱�ʾ�����+�۵��ɹ���TiO2������C��Cl2��Ӧ��ȡ��̬TiCl4���Ȼ�ѧ����ʽΪTiO2(s)+2Cl2��g��+C��s��=TiCl4��g��+ CO2(g)��H=-243KJ/mol��





