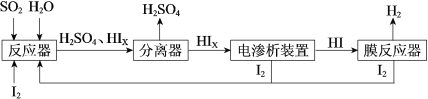
��Ŀ����
����Ŀ�����ݻ�Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2����500��ʱ������Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H<0��CH3OH��Ũ����ʱ��仯��ͼ��ʾ������˵������ȷ����
CH3OH(g)��H2O(g) ��H<0��CH3OH��Ũ����ʱ��仯��ͼ��ʾ������˵������ȷ����
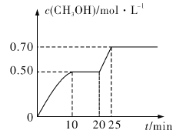
A.�ӷ�Ӧ��ʼ��10minʱ��H2��ƽ����Ӧ����v(H2)��0.15 mol/(L��min)
B.��20min��25min�ﵽ�µ�ƽ�⣬����������ѹǿ
C.�����������䣬���¶�����700�����ٴδ�ƽ��ʱƽ�ⳣ����С
D.�ӿ�ʼ��25min��CO2��ת������70%
���𰸡�B
��������
A������ͼ���֪��10minʱ�״���Ũ��Ϊ0.50mol��L��1����������Ũ�ȱ仯Ϊ0.50mol��L��1��3=1.5mol��L��1��H2��ƽ����Ӧ����v(H2)��0.15 mol/(L��min)����A��ȷ��
B����20min��25min�ﵽ�µ�ƽ�⣬��Ϊ��ߵĻ�ѧ������֮�ʹ����ұߣ�����ѹǿ����CH3OH�ĺ��������ǵ�ѹǿ����Ũ��Ӧ�����������н�����̣���B����
C�������������䣬���¶�����700������ѧƽ�������ƶ�����Ӧ��Ũ����������Ũ�ȼ�С�����Ի�ѧƽ�ⳣ����С����C��ȷ��
D���ӿ�ʼ��25min���״���Ũ�ȱ仯Ϊ0.70mol��L��1�����ݶ���������ϵ��֪��������̼��Ũ�ȱ仯Ҳ��0.70mol��L��1��������̼��ת��Ϊ![]() =70%����D��ȷ��
=70%����D��ȷ��
��ѡB��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ������ϩ��![]() ���������������ϵ���Ҫ���壬��ͨ���ұ��������Ƶã�
���������������ϵ���Ҫ���壬��ͨ���ұ��������Ƶã�![]()
![]()
![]() ��H2(g)
��H2(g)
��1����֪��
��ѧ�� | C-H | C-C | C=C | H-H |
����/kJ/mol | 412 | 348 | 612 | 436 |
����������Ӧ����1mol��������ЧӦ___�������Ŷ���kJ��
��2����ҵ�ϣ�ͨ�����ұ���EB�������в���N2��ԭ�������ұ���N2�����ʵ���֮��Ϊ1�U10��N2�����뷴Ӧ�������Ʒ�Ӧ�¶�600�棬��������ϵ��ѹΪ0.1Mpa����������½��з�Ӧ���ڲ�ͬ��Ӧ�¶��£��ұ���ƽ��ת���ʺ�ij���������±���ϩ��ѡ���ԣ�ָ����H2����IJ����б���ϩ�����ʵ���������ʾ��ͼ��
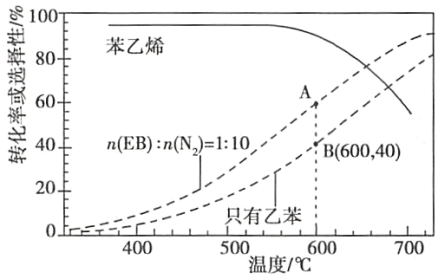
��A��B�����Ӧ������Ӧ���ʽϴ����___��
�ڿ��Ʒ�Ӧ�¶�Ϊ600���������___��