��Ŀ����
����Ŀ������������Ԫ��X��Y��Z��Q��R��ԭ������������������X�ĵ������ܶ���С�����壬Z��ͬ������ԭ�Ӱ뾶����Ԫ�أ�Y��Qͬ���壬��Q��ԭ��������Y���������ش��������⣺
(1)Z��ԭ�ӽṹʾ��ͼΪ__________��R��Ԫ�����ڱ��е�λ����______________��
(2)Z������������Ӧˮ����������ѧ��������_______________��Q��R������������Ӧ��ˮ�������Խ�ǿ����__________(�ѧʽ)��
(3)��X��Y�����γ�һ��Һ̬������T��T���Ӻ�18�����ӣ��������ʽΪ_______����Q�ĵͼ�������ͨ��T��������һ��ǿ�ᣬ��Ӧ�Ļ�ѧ����ʽΪ___________________��
(4)��Ԫ�ص�һ���⻯��M��M���Ӻ�18�����ӣ��ö��Ե缫��M��������Z������������Ӧˮ�������Һ���һ��ȼ�ϵ�أ���M�����ĵ缫��ӦʽΪ______________________��
(5)��2 L�ܱ������зֱ����4 mol A�����6 mol B���壬��һ�������·�����Ӧ��4A(g)��5B(g)![]() 4C(g)��xD(g)��5min��ﵽƽ��״̬�����ⶨB��ת����Ϊ75%��D��Ũ��Ϊ2.7 mol��L��1����x��_______��A��ƽ�������е��������Ϊ________���ӷ�Ӧ��ʼ��ƽ��ʱ����C��Ũ�ȱ仯��ʾ�÷�Ӧ��ƽ������v(C)��_____________��
4C(g)��xD(g)��5min��ﵽƽ��״̬�����ⶨB��ת����Ϊ75%��D��Ũ��Ϊ2.7 mol��L��1����x��_______��A��ƽ�������е��������Ϊ________���ӷ�Ӧ��ʼ��ƽ��ʱ����C��Ũ�ȱ仯��ʾ�÷�Ӧ��ƽ������v(C)��_____________��
���𰸡� �������ڢ�A�� ���Ӽ���(����)���ۼ� HClO4
�������ڢ�A�� ���Ӽ���(����)���ۼ� HClO4 ![]() SO2��H2O2==H2SO4 N2H4��4e�� +4OH- =N2+4H2O 6 3.7% 0.36 mol/(L��min)
SO2��H2O2==H2SO4 N2H4��4e�� +4OH- =N2+4H2O 6 3.7% 0.36 mol/(L��min)
��������
����������Ԫ��X��Y��Z��Q��R��ԭ������������������X�ĵ������ܶ���С�����壬��X��H��Z��ͬ������ԭ�Ӱ뾶����Ԫ�أ���Z��Na��Y��Qͬ���壬��Q��ԭ��������Y����������Y��O��Q��S�����ϣ�R��Cl��
(1) Z��Na��Na��ԭ������Ϊ11��ԭ�ӽṹʾ��ͼΪ ��R��Cl ��Cl��ԭ��������17����Ԫ�����ڱ��е�λ���ǵ������ڢ�A�壻
��R��Cl ��Cl��ԭ��������17����Ԫ�����ڱ��е�λ���ǵ������ڢ�A�壻
(2) Na������������Ӧˮ������NaOH�������ӻ����������ѧ�����������Ӽ���(����)���ۼ����ǽ�����Խǿ������������Ӧ��ˮ��������Խǿ���ǽ����ԣ�S<Cl��������������Ӧ��ˮ�������Խ�ǿ����HClO4��
(3)��H��O�γ�һ��18�����ӵ�Һ̬������H2O2���ǹ��ۻ����H2O2����ʽΪ![]() ����S�ĵͼ�������SO2��ͨ��H2O2��������ǿ��H2SO4����Ӧ�Ļ�ѧ����ʽΪSO2��H2O2==H2SO4��
����S�ĵͼ�������SO2��ͨ��H2O2��������ǿ��H2SO4����Ӧ�Ļ�ѧ����ʽΪSO2��H2O2==H2SO4��
(4)��Ԫ�غ�18�����ӵ��⻯��N2H4���ö��Ե缫��N2H4��������NaOH��Һ��ɵ�ȼ�ϵ�أ�N2H4�����ϼ�����ʧȥ���ӷ���������Ӧ���缫��ӦʽΪN2H4��4e�� +4OH- =N2+4H2O��
(5) 5min��ﵽƽ��״̬�����ⶨB��ת����Ϊ75%������n��B��=6 mol![]() 75%=4.5 mol����c��B��=
75%=4.5 mol����c��B��=![]() =
=![]() =2.25 mol��L��1����c��D��=2.7 mol��L��1�������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȵã�B��D���ʵ����仯��֮��5:6����x��6��
=2.25 mol��L��1����c��D��=2.7 mol��L��1�������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȵã�B��D���ʵ����仯��֮��5:6����x��6��
4A(g)��5B(g)![]() 4C(g)��6D(g)
4C(g)��6D(g)
n��ʼ��mol�� 4 6 0 0
��n��mol�� 3.6 4.5 3.6 5.4
nƽ�⣨mol�� 0.4 1.5 3.6 5.4
A��ƽ�������е��������=![]() 3.7%��
3.7%��
�ӷ�Ӧ��ʼ��ƽ��ʱ����C��Ũ�ȱ仯��ʾ�÷�Ӧ��ƽ������v(C)��![]() =
=![]() =
=![]() 0.36 mol/(L��min)��
0.36 mol/(L��min)��

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д�����Ŀ���״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ�� CO(g)+ 2H2(g)![]() CH3OH(g) ��H1����116 kJ��mol��1
CH3OH(g) ��H1����116 kJ��mol��1
��1����֪��![]() ��H2����283 kJ��mol��1
��H2����283 kJ��mol��1
![]() ��H3����242 kJ��mol��1
��H3����242 kJ��mol��1
���ʾ1mol��̬�״���ȫȼ������CO 2��ˮ����ʱ���Ȼ�ѧ����ʽΪ��
_______________________________________________��
��2�����ݻ�Ϊ1L�ĺ��������У��ֱ��о���230�桢250�桢 270�������¶��ºϳɼ״��Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��CO����ʼ��ɱȣ���ʼʱCO�����ʵ�����Ϊ1mol����COƽ��ת���ʵĹ�ϵ����ش���
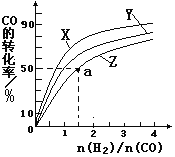
�������������¶��У�����Z��Ӧ���¶���__________
������ͼ��a���Ӧ�����ݣ����������Z�ڶ�Ӧ�¶���CO(g)+ 2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��K=_____________________��
CH3OH(g)��ƽ�ⳣ��K=_____________________��
��3����ij�¶��£���һ������CO��H2Ͷ��10L���ܱ������У�5minʱ�ﵽƽ�⣬�����ʵ����ʵ�Ũ��(molL-1)�仯���±���ʾ��
0min | 5min | 10min | |
CO | 0.1 | 0.05 | |
H2 | 0.2 | 0.2 | |
CH3OH | 0 | 0.04 | 0.05 |
��5min��10minֻ�ı���ijһ���������ı��������_________________________���Ҹ��������ı������_______________��
����Ŀ�����ж������������ݵ���ؽ�����ȷ���ǣ� ��
ѡ�� | ���� | ���� |
A | ��ɰ��֮��ˮ���������ֻ��ɵ�ɰ | ������Ӧ��Ϊ���淴Ӧ |
B | ���������ߣ���ض����࣬��ȡ��ɳճ����Ϊ֮ | �������ߡ�����Ҫ�ɷ�Ϊ������ |
C | ��ʯ��KNO3��������ѩ������ǿ��֮���������� | �����������̡���ԭ��ΪKNO3�ֽ� |
D | �䷨��Ũ�ƾ��Ͳ���ƿ���������ϣ������е�¶ | �����漰�IJ�������Ϊ����Ũ�� |
A. AB. BC. CD. D
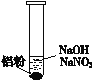
����Ŀ��һλͬѧ�ڸ�ϰʱ��������һ��ϰ�⣺ij��ɫ��Һ�п��ܺ�����H+��OH-��Na+��NO3-�����������ۺ�ֻ����H2���ʸ���ɫ��Һ���ܴ��������ļ������ӡ�
��1���������۲���H2��˵��������__________��������������������ԭ��������
��2����ͬѧ��H+�������ڣ���NO3-�Ͳ��ܴ������ڡ����ʵ��֤ʵ���£�
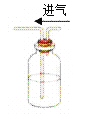
װ �� | �� �� |
| ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ�Һ���Ϸ���dz��ɫ ��. �Թܱ��ȣ���Һ���� |
�������ܽ�Al2O3��Ĥ�����ӷ���ʽ��_______________________________��
�ڸ������������Ʋ���Һ�в�����NO��Ϊ��һ��ȷ�ϣ���������ʵ�飺
ʵ�� | ���� | ���� |
ʵ��1 | ��ʪ��KI��������ֽ���ڿ����� | δ���� |
ʵ��2 | ��ʪ��KI��������ֽ����dz��ɫ���� | ��ֽ���� |
a��dz��ɫ������____________��
b��ʵ��1��Ŀ����_______________________________��
c��ʵ��1��2˵����Ӧ������NO��������NO�����ӷ���ʽ����������
______Al +______NO3-+_____ _ ==______Al 3++______NO+______ ��_______
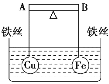
��3���ټ��裺��OH-�������ڣ�NO3-Ҳ���ܲ��ܴ������ڡ��������ʵ��֤ʵ���£�
װ �� | �� �� |
| ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ��д̼�����ζ |
Ϊȷ�����̼�����ζ�����壬��������ʵ�飺��ʪ��KI��������ֽ���飬δ��������ʪ���ɫʯ����ֽ���飬��ֽ������
�ٴ̼�����ζ��������____________��
�ڲ�������������ӷ���ʽ��____________________________________��
��4����NaOH��Һ�м������ۣ����ֻ�������H2���ɣ��仯ѧ����ʽ��_________________________________��
��5��ʵ����֤ʵ��NO3-���ᡢ���Ի����ж���һ���������ԣ������������ʣ�������������������е���ɫ��Һһ���ܴ������ڵ���_______________��