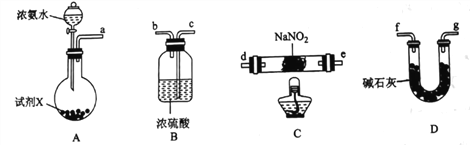
ЬтФПФкШн
ЁОЬтФПЁПЕўЕЊЛЏФЦ(NaN3)ПЩгУгкЦћГЕАВШЋЦјФвЕФЬэМгМСЃЌгыЫсЗДгІЩњГЩN2КЭH2ЁЃФГбЇЯАаЁзщдкЪЕбщЪвгУбЧЯѕЫсФЦКЭАБдкЮобѕЛЗОГжажЦБИNaN3ЃЌЩшМЦШчЯТЪЕбщ(МаГжзАжУТдШЅ):
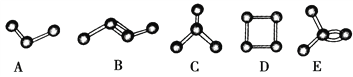
ЃЈ1ЃЉАДЦјСїДгзѓЕНгвЕФЗНЯђЃЌЩЯЪізАжУКЯРэЕФСЌНгЫГађЮЊ_________________(ЬювЧЦїНгПкзжФИ)ЁЃ
ЃЈ2ЃЉЪдМСXПЩбЁгУ________________(ЬюЪдМСУћГЦ)ЁЃ
ЃЈ3ЃЉзАжУCжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________________________________________ЃЌИУЗДгІашвЊдкЮобѕЛЗОГжаНјааЕФдвђЮЊ_______________________________________________ЁЃ
ЃЈ4ЃЉзАжУBЕФзїгУЮЊ___________________________________________ЁЃ
ЃЈ5ЃЉМгШШЬѕМўЯТЃЌNaNH2КЭN2OЗДгІвВПЩжЦБИNaN3ЃЌЭЌЪБЩњГЩФмЪЙЪЊЯьЕФКьЩЋЪЏШяЪджНБфРЖЕФЦјЬхЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________________________________________ЁЃ
ЃЈ6ЃЉHNO2ЪЧвЛдЊШѕЫсЃЌВЛЮШЖЈЃЌШнвзЗжНтЮЊСНжжЕЊЕФбѕЛЏЮяЁЃЯжгУШчЯТЪдМСЃКNaNO2ЁЂpHЪджНЁЂЫЎЁЂЯЁСђЫсЁЂNaOHШмвКЁЂМюЪЏЛвЁЂCuSO4ЁЂCuOЃЌМЦЪЕбщжЄУї((ПЩМгШШ)ЃЛ
ЂйHNO2ЮЊШѕЫс:____________________________________________________ЁЃ
ЂкзАжУCжаЗДгІНјаавЛЖЮЪБМфКѓЃЌгаNaN3ЩњГЩЃК______________________________________ЁЃ
ЁОД№АИЁПafg(Лђgf)de(Лђed)c(b) МюЪЏЛв(ЛђЩњЪЏЛвЛђNaOHЙЬЬх) 5NH3+4NaNO2![]() 3NaN3+NaOH+7H2O МгШШЬѕМўЯТЃЌNaNO2ШнвзБЛбѕЦјбѕЛЏ ЗРжЙЭтНчПеЦјНјШЫCзАжУИЩШХЪЕбщВЂЮќЪеРДВЮМгЗДгІЕФАБЦј 2NaNH2+N2O
3NaN3+NaOH+7H2O МгШШЬѕМўЯТЃЌNaNO2ШнвзБЛбѕЦјбѕЛЏ ЗРжЙЭтНчПеЦјНјШЫCзАжУИЩШХЪЕбщВЂЮќЪеРДВЮМгЗДгІЕФАБЦј 2NaNH2+N2O![]() NaN3+NaOH+NH3 НЋNaNO2ШмгкЪЪСПЫЎжаХфГЩШмвКЃЌГЃЮТЯТЃЌгУpHЪджНВтЖЈИУШмвКЕФpH>7 ШЁЩйСПзАжУCжаЙЬЬхМгШыЯЁСђЫсжаЃЌНЋВњЩњЕФЦјЬхЭЈЙ§зуСПЕФNaOHШмвКЃЌОМюЪЏЛвИЩдяКѓЭЈЙ§зЦШШЕФCuOЃЌдйНЋЩњГЩЕФЦјЬхЭЈЙ§CuSO4ЃЌШєЙлВьЕНCuOКкЩЋЙЬЬхБфКьЃЌCuSO4БфРЖЃЌдђгаNaN3ЩњГЩ
NaN3+NaOH+NH3 НЋNaNO2ШмгкЪЪСПЫЎжаХфГЩШмвКЃЌГЃЮТЯТЃЌгУpHЪджНВтЖЈИУШмвКЕФpH>7 ШЁЩйСПзАжУCжаЙЬЬхМгШыЯЁСђЫсжаЃЌНЋВњЩњЕФЦјЬхЭЈЙ§зуСПЕФNaOHШмвКЃЌОМюЪЏЛвИЩдяКѓЭЈЙ§зЦШШЕФCuOЃЌдйНЋЩњГЩЕФЦјЬхЭЈЙ§CuSO4ЃЌШєЙлВьЕНCuOКкЩЋЙЬЬхБфКьЃЌCuSO4БфРЖЃЌдђгаNaN3ЩњГЩ
ЁОНтЮіЁП
(1)гЩЪЕбщФПЕФКЭдРэПЩжЊЃЌзАжУAгУгкжЦАБЦјЃЌзАжУDгУгкИЩдяАБЦјЃЌзАжУCгУгкжЦNaN2ЃЌзАжУBПЩгУгкЗРжЙЭтНчПеЦјНјШыгкШХЪЕбщВЂЮќЪеЮДВЮМгЗДгІЕФАБЦјЁЃЙЪАДЦјСїДгзѓЕНгвЕФЗНЯђЃЌЩЯЪізАжУКЯРэЕФСЌНгЫГађЮЊafg(Лђgf)(Лђed)cЁЃ
(2)зАжУAЪЧгУХЈАБЫЎжЦАБЦјЃЌЙЪПЩбЁгУМюЪЏЛвЛђЩњЪЏЛвЛђNaOHЙЬЬх
(3)зАжУCжаЗЂЩњNH3гыNaNO2дкМгШШЬѕМўЯТжЦNaN3ЕФЗДгІЃЌИљОнЕУЪЇЕчзгЪиКуКЭдзгЪиКуЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ5NH3+4NaNO2+3NaN3+NaOH+7H2OЁЃМгШШЬѕМўЯТЃЌNaNO2ШнвзБЛбѕЦјбѕЛЏЃЌЙЪИУЗДгІашдкЮобѕЛЗОГЯТЗДгІ
(4)зАжУBПЩгУгкЗРжЙЭтНчПеЦјНјШыИЩШХЪЕбщВЂЮќЪеЮДВЮМгЗДгІЕФАБЦјЁЃ
(5)гЩаХЯЂЃЌМгШШЬѕМўЯТЃЌNaNH2КЭN2OЗЂЩњбѕЛЏЛЙдЗДгІжЦБИNaNЃЌИљОнЕУЪЇЕчзгЪиКуКЭдзгЪиКужЊЃЌЗДгІЗНГЬЪНЮЊ2NaNH2+N2O![]() NaN3+NaOH+NH3ЁЃ
NaN3+NaOH+NH3ЁЃ
(6)ЂйжЄУїФГЫсЮЊШѕЫсПЩгУЦфЖдгІбЮЫЎНтЪЙвКМюадЛђВтЖЈвЛЖЈХЈЖШЕФИУЫсШмвКЕФpHЫЕУїИУЫсВЛЭъШЋЕчРыРДЪЕЯжЂкгЩаХЯЂЃЌNaN3гыЫсЗДгІЩњГЩN2КЭH2ЃЛHNO2ЪЧвЛдЊШѕЫсЃЛВЛЮШЖЈЃЌШнвзЗжНтЮЊСНжжЕЊЕФбѕЛЏЮяЁЃдђжЄУїзАжУCжаЗДгІНјаавЛЖЮЪБМфКѓЃЌВПЗжNaNO2гаNaN3ЩњГЩЕФЗНЗЈЮЊШЁЩйСПзАжУCжаЙЬЬхМгШыЯЁСђЫсжаЃЌНЋВњЩњЕФЦјЬхЭЈЙ§зуСПЕФNaOHШмвКЃЌОМюЪЏЛвИЩдяКѓЭЈЙ§зЦШШЕФCuOЃЌдйНЋЩњГЩЕФЦјЬхЭЈЙ§CuSO4ЃЌШєЙлВьЕНCuOКкЩЋЙЬЬхБфКьЃЌCuSO4БфРЖЃЌдђгаNaN3ЩњГЩЁЃ

 УћЪІжИЕМЦкФЉГхДЬОэЯЕСаД№АИ
УћЪІжИЕМЦкФЉГхДЬОэЯЕСаД№АИ ПЊаФЭмПкЫуЬтПЈЯЕСаД№АИ
ПЊаФЭмПкЫуЬтПЈЯЕСаД№АИ