��Ŀ����
����Ŀ��ij��ȤС���������Ʊ�![]() ��
��![]() ���������壬�����������£�
���������壬�����������£�

��֪��������һ�ֹ��˵ķ�ʽ��
��ش�
(1)������еĻ�ѧ����ʽ___________��
(2)�����������![]() �����ӷ���ʽ_____________��
�����ӷ���ʽ_____________��
(3)д��![]() �ĵ��뷽��ʽ_____________��
�ĵ��뷽��ʽ_____________��
(4)������������������______��
A. ����ϴ����������ɣ����ɼ���![]() ����
����
B. Ϊ�˵õ���![]() ��������������
��������������
C. ��������ǯ�ƶ����ȵ���������Ԥ������ǯ
D. ����ȡ�º����ʯ��������ȴ����
E. Ϊȷ������ȷ��ȼ�պ�Ӧ���ȳ���
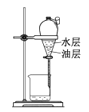
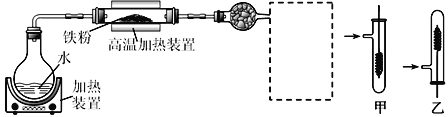
(5)����ҺA�Ʊ�![]() ��װ����ͼ��
��װ����ͼ��

��ͨ��HCl��������___________�ͽ���![]() ���ܽ�ȣ�ʹ����_______(�ѧʽ)������ʽ������
���ܽ�ȣ�ʹ����_______(�ѧʽ)������ʽ������
���ձ���NaOH��Һ��������___________������©����������__________________��
���𰸡�2Al��2NaOH��2H2O=2NaAlO2��3H2�� AlO![]() ��CO2��2H2O=Al(OH)3����HCO
��CO2��2H2O=Al(OH)3����HCO![]() Al3++3OH-
Al3++3OH-![]() Al(OH)3
Al(OH)3![]() H++AlO2-+H2O BCD ����AlCl3��ˮ�� AlCl3��6H2O ����HClβ�� ������
H++AlO2-+H2O BCD ����AlCl3��ˮ�� AlCl3��6H2O ����HClβ�� ������
��������
�������̣���NaOH��Һ�ܽ�������������Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2��������NaAlO2����Һͨ�������CO2���壬������Ӧ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-���õ�Al��OH��3�Ľ�״���壬��һ���ֽ�״����ϴ�ӵõ�Al��OH��3������Al��OH��3�õ����������������ܽ�Al��OH��3������K2SO4��Һ�����Ƹ�������10��20�������������Һ��ѡ��������С���岢��������Һ���룬��Ȼ��ȴ�����£��õ��������壬
��һ���ֽ�״�����������ܽ⣬�õ�AlCl3��Һ����HCl����ͨ��AlCl3��Һ������AlCl3ˮ��ʹٽ�AlCl36H2O�ᾧ����ˮԡ���ò�����ά���ˣ���Ũ����ϴ�Ӿ��壬��ֽ���ɸ����AlCl36H2O���壬�ݴ˷�������
��1���������ΪNaOH��Һ�ܽ�������������Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2�������ΪNaAlO2����Һ�������CO2��������Al(OH)3�����ӷ�ӦΪ��AlO2-+CO2+2H2O=Al(OH)3��+HCO3-���ʴ�ΪAlO2-+CO2+2H2O=Al(OH)3��+HCO3-��
��3��![]() ����������������ܷ�����ʽ���룬Ҳ�ܷ�����ʽ���룬���뷽��ʽΪ��Al3++3OH-
����������������ܷ�����ʽ���룬Ҳ�ܷ�����ʽ���룬���뷽��ʽΪ��Al3++3OH-![]() Al(OH)3
Al(OH)3![]() H++AlO2-+H2O���ʴ�Ϊ��Al3++3OH-
H++AlO2-+H2O���ʴ�Ϊ��Al3++3OH-![]() Al(OH)3
Al(OH)3![]() H++AlO2-+H2O��
H++AlO2-+H2O��
��4��A������ϴ������Ҫ���ɣ���������ʱ��ˮ���λ��������λ���¶Ȳ�һ��ʹ�������Ȳ����������ѿ�����A����
B��Ϊ�˵õ�������Al2O3�������������أ�ʹAl(OH)3��ȫ�ֽ⣬��B��ȷ��
C�������ڼ��Ⱥ��¶ȸߣ�������ǯΪ���£���ֱ�ӽӴ�����ʹ�������Ȳ����������ѿ�������Ԥ������ǯ����C��ȷ��
D������ȡ�º�Ӧ����ʯ��������ȴ����ֱ�ӷ��������ϣ���ʹ�������Ȳ����������ѿ�����D��ȷ��
E��Al(OH)3���պ�Ӧ��ȴ����أ���E���ʴ�Ϊ��BCD��
��5����Al3+�ᷢ��ˮ�ⷴӦ��Al3++3H2OAl��OH��3+3HCl��ͨ��HCl��������Al3+��ˮ�⣬��������Һ��Cl-��Ũ�ȣ�ʹ����AlCl36H2O��ʽ�ᾧ���ʴ�Ϊ������AlCl3��ˮ�⣻AlCl3��6H2O��
���ձ���NaOH��Һ������������HClβ��������©���������Ƿ�ֹ�������ʴ�Ϊ������HClβ����������