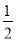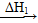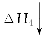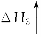��Ŀ����
Ŀǰ�����о�����Դ��ǰ�ؼ����ϣ���ѧ�������ڶ�����̼�������ת�����������о����ѹ���Ķ�����̼ת��Ϊ��������������ʡ�
(1)��ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־֮һ������ʯ�ͻ��������������ϩ�IJ�������________��
A�������� B�����ѻ�
C�����ѽ� D��������
(2)����˵������ȷ����__________��
A��ʯ���ǻ��������õ�������Ǵ�����
B��ú����������ǿ�ȿ��Եõ��ں�ɫ��״��ú����
C��ú��������Һ����ʹú��������Դ����Ч;��
D������ɫ��ѧ�Ƕȿ��ǣ���ֲ��Ϊ������������Դ����δ����������Դ
(3)�����CO2��H2��1��4�ı�����ϣ�ͨ�뷴Ӧ�������ʵ��������·�Ӧ���ɻ��һ����Ҫ����Դ����������»�ѧ����ʽ��
CO2��4H2�D��(__________)��2H2O
(4)����CO2��H2��1��3�ı�����ϣ�ʹ֮������Ӧ����ij����Ҫ�Ļ���ԭ�Ϻ�ˮ����ԭ�Ͽ�����________��
A������ B��ϩ�� C��Ȳ�� D��������
(5)�ס��ҡ����������ʳ������������õ�ԭ�ϲ�ͬ��������������ͬ��
ԭ����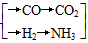 ��CO(NH2)2
��CO(NH2)2
���׳��Խ�̿��ˮΪԭ�ϣ����ҳ�����Ȼ����ˮΪԭ�ϣ���������ʯ����(��Ҫ����ΪC5H12)��ˮΪԭ�ϡ�����ҵ�йع涨������ԭ�����Ƶõ�ԭ����H2��CO2�����ʵ���֮�ȣ�����ӽ��ϳ����ص�ԭ����NH3(�����H2�����ʵ���)��CO2�����ʵ���֮�ȣ����ԭ�ϵ���������ߡ��ݴ��жϼס��ҡ������������ĸ�������ԭ�ϵ���������ߣ�________��
(6)�����������Ϣ����ƹ�ҵ�ϳ����ص�����_________________________��
(1)C��(2)A��(3)CH4��(4)B
(5)�ҡ�(6)��ѹ�����������˵��¶�
��������(1)ʯ�������������������������ķ������γɵĻ�����������õ��IJ���һ����ʯ���к��е����ʣ���Ϊ�����������仯����Ҫ��ʯ���еõ���ϩ�����ʯ�Ͳ�Ʒ�����ѽ⣬������ѧ��Ӧ��õ�����ϩ�����ĸߵ;���һ������ʯ�ͻ���ˮƽ�ĸߵ͡�
(2)ʯ�ͷ���õ��IJ�����һ���¶ȷ�Χ�����ʣ����ԣ�����������ﻹ�ǻ���ú����������ǿ�ȿ��Եõ�����Ľ�̿���ں�ɫ��״��ú���͡��ְ�ˮ�ͽ�¯ú��������ú�����IJ����к���ú���ͺʹְ�ˮ��Ϊ�����ú��ȼ��Ч�ʣ���ú������Һ����ʹú���������Դ��ֲ��ȼ�յIJ���ΪCO2��H2O����CO2��H2O���ܱ�ֲ�����գ������������ת����ֲ���ڵ����࣬������ɫ��ѧ��˼�롣
(3)���ݻ�ѧ����ʽ���غ�ԭ���ɵõ��𰸡�
(4)����(3)��˼·��Ӧ��ԭ���غ�д����Ӧ��ѧ����ʽΪ��CO2��3H2�D��2H2O��CH2�����з���ʽ����CH2����Ϊϩ������2CO2��6H2�D��4H2O��C2H4��3CO2��9H2�D��6H2O��C3H6��
(5)д���������صĻ�ѧ����ʽΪCO2��2NH3�D��CO(NH2)2��H2O����N2��3H2 2NH3���õ��ϳ����ص�ԭ����NH3(�����H2�����ʵ���)��CO2�����ʵ���֮��Ϊ3��1�����2CO��O2=2CO2���ó���Ӧ���ɵ�CO��H2�����ʵ�����Ϊ1��3ʱ���ϳɵõ��϶�����أ���֤��õ���Ϊ�ҳ���
2NH3���õ��ϳ����ص�ԭ����NH3(�����H2�����ʵ���)��CO2�����ʵ���֮��Ϊ3��1�����2CO��O2=2CO2���ó���Ӧ���ɵ�CO��H2�����ʵ�����Ϊ1��3ʱ���ϳɵõ��϶�����أ���֤��õ���Ϊ�ҳ���