��Ŀ����
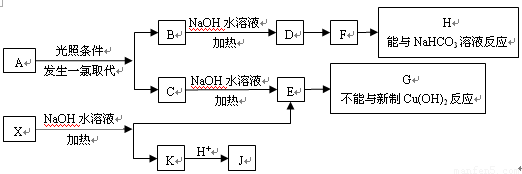
(15��) ��֪ij�л���A������ͼ��ʾ����Է�������Ϊ84����
(1) ��A�ķ���ʽΪ_____________��
(2) ����A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ__________________��
(3) ����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ��ʽΪ ��
(4) ����A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ����________��ͬ���칹�塣
(5)����A������ױ��������к���̼̼˫�����˴Ź����ױ���������ֻ��һ�����͵��⡣����ͼ�У�D1��D2��Ϊͬ���칹�壬E1��E2��Ϊͬ���칹�塣
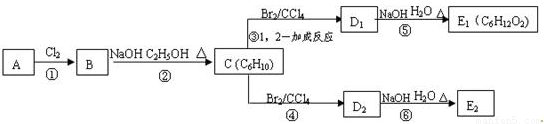
��Ӧ�ڵĻ�ѧ����ʽΪ ______________________ ��C�Ļ�ѧ����Ϊ ___________ ��
E2�Ľṹ��ʽ�� ________________ ���ķ�Ӧ������ _____________ ��
��15�֣��� C6H12�� ��2�֣� (2)  �� ��2�֣�
�� ��2�֣�
(3) (CH3)3CCH=CH2��CH2=C(CH3)CH(CH3)2�� (CH3)2C=C(CH3)2�� ��3�֣�
(4) 5 (2��)
��5��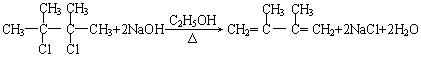 ��2�֣�
��2�֣�
2��3-����-1��3-����ϩ��1�֣�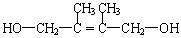 ��2�֣�
ȡ����Ӧ����1�֣�
��2�֣�
ȡ����Ӧ����1�֣�
����������

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�