��Ŀ����
��ʯ���ѽ����ϩ��Ϊԭ�Ϻϳ�һЩ�����ʵ�·�����¡�
��֪��Diels-Alder��ӦΪ����˫ϩ�뺬��ϩ����Ȳ���Ļ����������������Ԫ��״������ķ�Ӧ�����Diels-Alder��Ӧ��

���������գ�
��1��д��X���������������ŵ����� ��
��2��д��A��B�Ļ�ѧ����ʽ ��
��3��д�������ʵ����� ��
��4�����������ҷ����к�����������Y��ͬ���칹���� �֡�
��5��R��W��һ��ͬ���칹�壬R��FeCl3��Һ����ɫ����R������Ũ��ˮ��Ӧ��д��R�Ľṹ��ʽ ��
��6��д��ʵ������D�Ʊ�E�ĺϳ�·�ߡ�
���ϳ�·�߳��õı�ʾ��ʽΪ�� ��
��
��1����ԭ�� ̼̼˫��
��2��ClCH2-CH2CH2CH2Cl+2NaOH=������=CH2=CH-CH=CH2+2NaCl+2H2O
��3�������ϩ
��4��3
��5�����ǻ����ڶ�λ���й����ţ���ĿӦ�ö�һ������ֻ��������ԭ�ӣ�
��6���������ӳ���NaOH��Һ��ȥ
���������������1����������Ϣ�ڹ��������½��еķ�ӦӦ��ȡ����Ӧ��X�Ľṹ��ʽΪCH2Cl-CH=CH-CH2Cl ����������������ԭ�Ӻ�̼̼˫����A�Ľṹ��ʽΪClCH2-CH2CH2CH2Cl��
��2��A��B������ȥ��Ӧ������ʽΪClCH2-CH2CH2CH2Cl+2NaOH��CH2=CH-CH=CH2+2NaCl+2H2O����B�Ľṹ��ʽΪCH2=CH-CH=CH2��B��D����������Ϣ�ļӳɷ�Ӧ����D����Ŀ����߷������ʷ����䵥��EΪ ��
��
��3����X��Y��������֪��������ˮ�ⷴӦ��YΪCH2OH-CH=CH-CH2OH��ZΪ OHC-CH=CH-CHO,��Ŀ���ﲢ������Ϣ���Ƶü�Ϊ�����ϩ��WΪ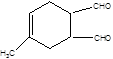 ��
��
��4��YΪCH2OH-CH=CH-CH2OH�����������ҷ����к�����������Y��ͬ���칹����CH3COOCH2CH3,HCOOCH(CH3)2,CH3CH2COOCH3��3�֡�
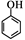
��5��R��W��һ��ͬ���칹�壬˵������ʽ��ͬ��R��FeCl3��Һ����ɫ��˵���Ƿ������ʣ���R������Ũ��ˮ��Ӧ����֪���ǻ��ڶ�λû����ԭ�ӣ���R�Ľṹ��ʽ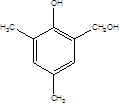 ,�����������֣����ǻ����ڶ�λ���й����ţ���ĿӦ�ö�һ������ֻ��������ԭ�ӣ���
,�����������֣����ǻ����ڶ�λ���й����ţ���ĿӦ�ö�һ������ֻ��������ԭ�ӣ���
��6���������ӳ���NaOH��Һ��ȥ���ϳ�·��Ϊ ��
��
���㣺������Ҫ�����л���ṹ��ʽ���ƶϣ�ͬ���칹����жϣ��л��ϳ�·�ߵ���д��

 ��У����ϵ�д�
��У����ϵ�д�

 ��________(д�ṹ��ʽ)����
��________(д�ṹ��ʽ)����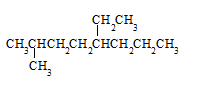
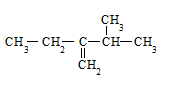



 ����д�����ڼ����ӣ�
����д�����ڼ����ӣ�  �����Ҵ�Ϊԭ���Ʊ�
�����Ҵ�Ϊԭ���Ʊ�  �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���

 ����CH2��CH2Ϊԭ���Ʊ��л���
����CH2��CH2Ϊԭ���Ʊ��л��� �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���

 ��DMC1��1�������Ʒ�Ӧ�ٵķ�Ӧ����д����Ӧ����ʽ ��
��DMC1��1�������Ʒ�Ӧ�ٵķ�Ӧ����д����Ӧ����ʽ �� B+C���йأõ�ת����ϵ����ͼ��ʾ��
B+C���йأõ�ת����ϵ����ͼ��ʾ��
