��Ŀ����
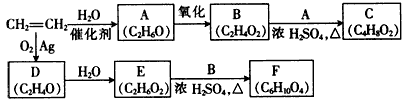
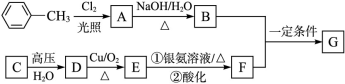
��֪�л���A��B��C��D��E��F��G������ת����ϵ,����C�IJ�������������һ�����ҵ�ʯ�ͻ�����չˮƽ,G�ķ���ʽΪC9H10O2,�Իش������й����⡣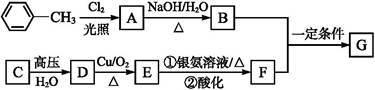
(1)G�Ľṹ��ʽΪ������������
(2)ָ�����з�Ӧ�ķ�Ӧ����:Aת��ΪB:������,Cת��ΪD:������������
(3)д�����з�Ӧ�Ļ�ѧ����ʽ:
D����E�Ļ�ѧ����ʽ:��������������������������
B��F����G�Ļ�ѧ����ʽ:����������������������
(4)��������������G��ͬ���칹����������:�ٱ�������3��ȡ����,��������ȡ������ͬ;���ܹ������Ƶ�������Һ��Ӧ����������������ԭ�ӹ������ֲ�ͬ�������������ʵĽṹ��ʽΪ��������������������������
(1)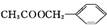
(2)ȡ����Ӧ���ӳɷ�Ӧ
(3)2CH3CH2OH+O2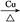 2CH3CHO+2H2O��CH3COOH+
2CH3CHO+2H2O��CH3COOH+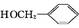

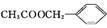 +H2O
+H2O
(4)6��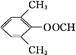 ��
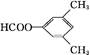
��
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
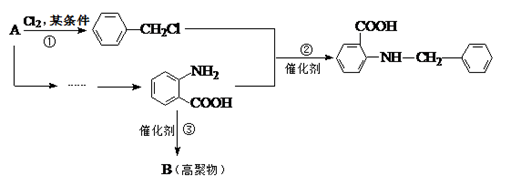
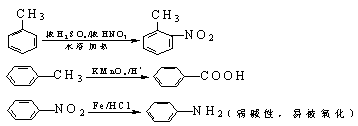
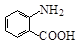 �й����ŵ����� ��
�й����ŵ����� ��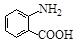 ��һ��̼��ͬϵ�����������������D��ͬ���칹�干�� �֣�д��һ�����������Һ�4�ֲ�ͬ��ԭ�ӵ�ͬ���칹��Ľṹ��ʽ ��
��һ��̼��ͬϵ�����������������D��ͬ���칹�干�� �֣�д��һ�����������Һ�4�ֲ�ͬ��ԭ�ӵ�ͬ���칹��Ľṹ��ʽ ��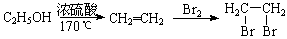 ��
��
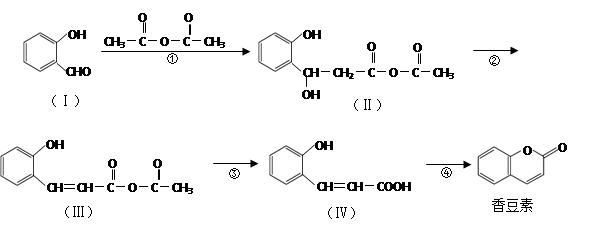
 ����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ����___________________________________________________��
����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ����___________________________________________________�� ���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����
���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����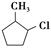 Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�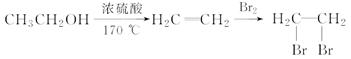

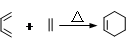

 ��
��
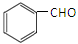 ��CH3CHO�ܷ������Ʒ�Ӧ�١��ڵ�������Ӧ���������ɵ��л���Ľṹ��ʽΪ ��
��CH3CHO�ܷ������Ʒ�Ӧ�١��ڵ�������Ӧ���������ɵ��л���Ľṹ��ʽΪ ��