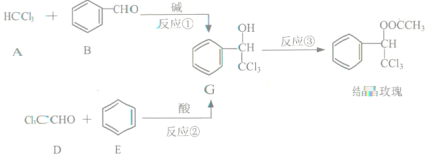
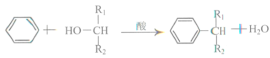
��Ŀ����
����Ŀ��NO��NO2�ɱ���ˮ��Һ���գ�6NO+ 4NH3��5N2+6H2O��6NO2+ 8NH3��7N2+12H2O��������NO��NO2������N2O4���������90mol��30%�İ�ˮ4.45��103g��ȫ���գ�����78mol���������պ�ˮ�ܶ�Ϊ0.980 g/cm3����
A.ԭ��������ƽ�����ΪNO1.1B.ԭ��ˮ��Ũ��ԼΪ17.3mol/L
C.���պ�ˮ��Ũ��ԼΪ2.4mol/LD.���պ�ˮ����������ԼΪ0.5
���𰸡�AC
��������
��Nԭ���غ����òμӷ�Ӧ�İ������ʵ�����n��NH3��+90mol=78mol��2��n��NH3��=66mol����ΪNO�����ʵ���x��NO2�����ʵ���Ϊ��90mol-x������66mol��3=xmol��2+��90-x��mol��4��x=81mol���������������ʵ���Ϊ=90mol-81mol=9mol���ݴ˽��
A��ԭ��������к���9molNO2��81molNO����ƽ����ԭ����Ϊ��![]() ����ԭ��������ƽ�����ΪNO1.1��A��ȷ��
����ԭ��������ƽ�����ΪNO1.1��A��ȷ��
B����������������ԭ��ˮ��Һ�������������ԭ��ˮ�����ʵ���Ũ�ȣ�B����
C��4.45��103g+81mol��30g/mol+9mol��46gmol-1-78mol��28g/mol=5110g����Ӧ����Һ���Ϊ��![]() ��5214mL=5.214L����Ӧ����Һ��ʣ�����ʰ������ʵ���=
��5214mL=5.214L����Ӧ����Һ��ʣ�����ʰ������ʵ���=![]() �����պ�ˮ�����ʵ���Ũ��=
�����պ�ˮ�����ʵ���Ũ��=![]() ��2.4 mol��L-1��C��ȷ��
��2.4 mol��L-1��C��ȷ��
D������C��֪�����պ�ˮ������Ϊ��5110g�����������ʵ���Ϊ12.53mol����ϡ�ͺ�ˮ����������Ϊ![]() ��D����
��D����
��ѡAC��

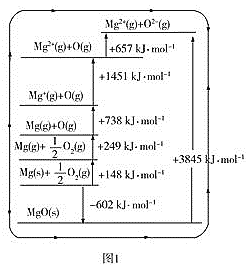
����Ŀ��Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
��1����ԭ������������˳��ϡ��������⣩������˵����ȷ����___��
a��ԭ�Ӱ뾶�����Ӱ뾶����С b�������Լ������ǽ�������ǿ
c�����ʵ��۵㽵�� d���������Ӧ��ˮ������Լ�����������ǿ
��2��ԭ�������������������������ͬ��Ԫ������Ϊ___�������������ļ���������___��
��3����֪��
������ | MgO | Al2O3 | MgCl2 | AlCl3 |
���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
�۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����___������ʱ�����Al2O3�������AlCl3��ԭ����___��
��4������裨�۵�1410���������õİ뵼����ϣ��ɴֹ��ƴ���������£�
Si���֣�![]() SiCl4
SiCl4![]() SiCl4������
SiCl4������![]() Si������
Si������
д��SiCl4�ĵ���ʽ��___����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12kg����������akJ������д���÷�Ӧ���Ȼ�ѧ����ʽ___��
��5���������岻����Ũ����������P2O5�������___��
a��NH3 b��HI c��SO2 d��CO2
��6��KClO3������ʵ������O2�������Ӵ�����400��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��___��
����Ŀ����������������ȷ���������ϵ����
ѡ�� | ���� | ������ |
A | �������ǿ�� | ������ܿ�ʴ���� |
B | ����Һ�� | Һ������������� |
C | ̼���Ƴ�������ķ��ݼ� | ̼���������ֽ� |
D | KNO3���ܽ�ȴ� | ���ؽᾧ����ȥKNO3�к��е�NaCl |
A.AB.BC.CD.D
����Ŀ������������ϡ���ױƷ��ҽҩ�����Ϻй���֬�ȵ���Ҫԭ�ϣ�ʵ���������з�Ӧ��ȡ����ᣮ
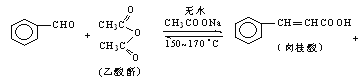 CH3COOH
CH3COOH
ҩƷ��������
����ȩ | ������ | ����� | ���� | |
�ܽ�ȣ�25�棬g/100gˮ�� | 0.3 | ����ˮˮ�� | 0.04 | ���� |
�е㣨�棩 | 179.6 | 138.6 | 300 | 118 |
��գ�
�ϳɣ���Ӧװ����ͼ��ʾ����������ƿ���Ⱥ������ϸ����ˮ�����ơ�����ȩ������������ʹ֮��Ͼ��ȣ� ��150��170�����1Сʱ��������״̬��
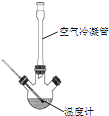
��1�����������ܵ�������__��
��2����װ�õļ��ȷ�����__�����Ȼ���Ҫ���Ʒ�Ӧ����״̬��������ҷ��ڣ��ᵼ���������ʽ��ͣ����ܵ�ԭ����______��
��3�������ô����ƾ��壨CH3COONa3H2O����ԭ����______��
��Ʒ���ƣ���������Ӧ��õ��Ļ������ȵ���Բ����ƿ�У��������в�����
��Ӧ�����![]()
![]()
![]()
![]()
![]() ����ᾧ��
����ᾧ��
��4���ӱ���Na2CO3��Һ����ת�����ᣬ��ҪĿ����_______��
��5������I��__������������ᾧ������Ȼ�����ʣ�����ߴ��ȿ��Խ��еIJ�����__������������ƣ���
��6�����ʵ�鷽�������Ʒ���Ƿ��б���ȩ_______��