��Ŀ����
(10��)ij�о���ѧϰС��Ϊ̽��Cu(OH)2���ȷֽ���P�������ʣ��������ʵ�顣
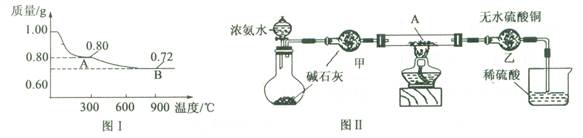
(1)ȡ0��98 g Cu(OH)2������ȣ��������¶ȱ仯��������ͼ1��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ �� ��
(2)ȡ��������B����������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�����л��к�ɫ������ڣ��÷�Ӧ�����ӷ���ʽΪ ��
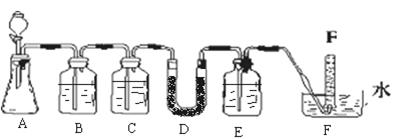
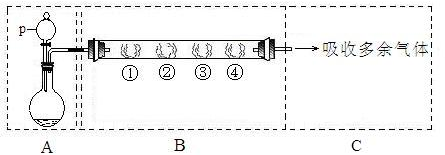
(3)Ϊ֤������A�ڼ���ʱ����NH3��Ӧ��ijͬѧ�������ͼ��(�г�װ��δ����)��ʾʵ��װ�á�
�ټ�������װ�������Եķ��� ��
��ʵ������й۲쵽������������ iֱ�������й����ɺ�ɫ��Ϊ��ɫ��iiװ�����й����ɰ�ɫ��Ϊ��ɫ����֤������A��NH3�����˷�Ӧ���ж����ݵ��� (��ѡ����ĸ)��
a��ֻ��i���� b��ֻ��ii���� c��i��ii������

(1)ȡ0��98 g Cu(OH)2������ȣ��������¶ȱ仯��������ͼ1��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ �� ��
(2)ȡ��������B����������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�����л��к�ɫ������ڣ��÷�Ӧ�����ӷ���ʽΪ ��
(3)Ϊ֤������A�ڼ���ʱ����NH3��Ӧ��ijͬѧ�������ͼ��(�г�װ��δ����)��ʾʵ��װ�á�
�ټ�������װ�������Եķ��� ��
��ʵ������й۲쵽������������ iֱ�������й����ɺ�ɫ��Ϊ��ɫ��iiװ�����й����ɰ�ɫ��Ϊ��ɫ����֤������A��NH3�����˷�Ӧ���ж����ݵ��� (��ѡ����ĸ)��
a��ֻ��i���� b��ֻ��ii���� c��i��ii������
��1��CuO Cu2O
��2��Cu2O + 2H+ = Cu2+ + Cu + H2O
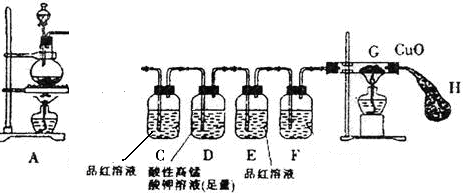
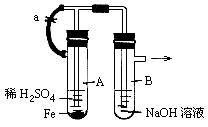
��3������ͼ����װ�ã��رշ�Һ©����������ĩ�˵��ܽ���ˮ�У�����ë����ס��ƿ�ײ��������ܿ�����������ð����������ë������ȴ�����º����γ�һ��Һ���ҳ������䣬��֤��װ�����������á�
��b
��2��Cu2O + 2H+ = Cu2+ + Cu + H2O
��3������ͼ����װ�ã��رշ�Һ©����������ĩ�˵��ܽ���ˮ�У�����ë����ס��ƿ�ײ��������ܿ�����������ð����������ë������ȴ�����º����γ�һ��Һ���ҳ������䣬��֤��װ�����������á�
��b
��1��������ˮ��ļ�Cu(OH)2�ɼ�����ˮ��Cu(OH)2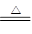 CuO��H2O���ɷ�Ӧǰ�����������ʵ�������Ϊ98��80��1.00��0.80�ɲ²��A����ΪCuO
CuO��H2O���ɷ�Ӧǰ�����������ʵ�������Ϊ98��80��1.00��0.80�ɲ²��A����ΪCuO
ͭ����������CuO��Cu2O��CuO��������������С�����ݷ�Ӧ��4CuO 2Cu2O+O2���ɿ�������Ӧǰ�����������ʵ�������Ϊ80��72���������⣬�ʿ�֪B����ΪCu2O
2Cu2O+O2���ɿ�������Ӧǰ�����������ʵ�������Ϊ80��72���������⣬�ʿ�֪B����ΪCu2O
��2��Cu2O������������������ͭ���ʼ�����ͭ����᪻���Ӧ��Cu2O + 2H+ = Cu2+ + Cu + H2O
��3���ٹرշ�Һ©����������ĩ�˵��ܽ���ˮ�У�����ë����ס��ƿ�ײ��������ܿ�����������ð����������ë������ȴ�����º����γ�һ��Һ���ҳ������䣬��֤��װ�����������á�
��������֪����CuO���ȿɷֽ����ɺ�ɫ��Cu2O���ʡ�ֱ�������й����ɺ�ɫ��Ϊ��ɫ��������˵��������CuO�μ��˷�Ӧ��
���ڰ������������ˮ��õ���ˮ�İ���������ˮ����ͭ������˵����ˮ���ɣ������е���Ԫ��ֻ�������ڰ������ʴ��������Ϊ�����μӷ�Ӧ������
bѡ���������
 CuO��H2O���ɷ�Ӧǰ�����������ʵ�������Ϊ98��80��1.00��0.80�ɲ²��A����ΪCuO
CuO��H2O���ɷ�Ӧǰ�����������ʵ�������Ϊ98��80��1.00��0.80�ɲ²��A����ΪCuOͭ����������CuO��Cu2O��CuO��������������С�����ݷ�Ӧ��4CuO
 2Cu2O+O2���ɿ�������Ӧǰ�����������ʵ�������Ϊ80��72���������⣬�ʿ�֪B����ΪCu2O
2Cu2O+O2���ɿ�������Ӧǰ�����������ʵ�������Ϊ80��72���������⣬�ʿ�֪B����ΪCu2O��2��Cu2O������������������ͭ���ʼ�����ͭ����᪻���Ӧ��Cu2O + 2H+ = Cu2+ + Cu + H2O
��3���ٹرշ�Һ©����������ĩ�˵��ܽ���ˮ�У�����ë����ס��ƿ�ײ��������ܿ�����������ð����������ë������ȴ�����º����γ�һ��Һ���ҳ������䣬��֤��װ�����������á�
��������֪����CuO���ȿɷֽ����ɺ�ɫ��Cu2O���ʡ�ֱ�������й����ɺ�ɫ��Ϊ��ɫ��������˵��������CuO�μ��˷�Ӧ��
���ڰ������������ˮ��õ���ˮ�İ���������ˮ����ͭ������˵����ˮ���ɣ������е���Ԫ��ֻ�������ڰ������ʴ��������Ϊ�����μӷ�Ӧ������
bѡ���������

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ

 2NH3(g)+CO2(g)
2NH3(g)+CO2(g)
 ʱ��0-6min ���������ˮ�ⷴӦ��ƽ������ ______��
ʱ��0-6min ���������ˮ�ⷴӦ��ƽ������ ______��