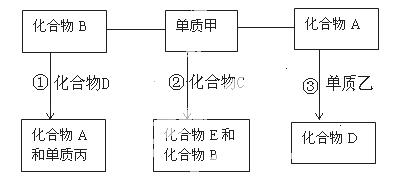
��Ŀ����
��ͼ��һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʱ���ȥ����Ӧ�ٳ���Ӧ����Ұ�⺸�Ӹֹ죬���ǹ�ҵ����Ҫ�ķ�Ӧ֮һ��
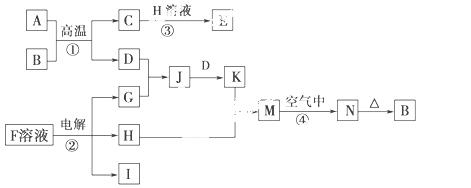
��ش��������⣺
(1)H�ĵ���ʽ��________________�����к��еĻ�ѧ��������___________
_________________��
(2)д����Ӧ�ܵ�����_____________________________________________
__________________________________________��
�йط�Ӧ�Ļ�ѧ����ʽΪ____________________________________________
___________________________��
(3)��֪I��ȼ�����ǣ�285.8 kJ��mol��1����1 m3(��״��)I��ȫȼ�գ��ָ�������ʱ�ų���������________(����������3λ��Ч����)��
(4)25 ��ʱ����PtΪ�缫��⺬���� ����̪��F�ı�����Һ������________(�����������)��������Һ����ɫ��Ϊ��ɫ�����ڴ˼��ռ���0.2 g���壬���ʱ��Һ��pH��________(������Һ�����Ϊ2 L�Ҳ����ǵ�����Һ����ı仯)��
����̪��F�ı�����Һ������________(�����������)��������Һ����ɫ��Ϊ��ɫ�����ڴ˼��ռ���0.2 g���壬���ʱ��Һ��pH��________(������Һ�����Ϊ2 L�Ҳ����ǵ�����Һ����ı仯)��
(5)��K��Һ�м�����K�����ʵ�����Na2O2��ǡ��ʹKת��ΪN��д���÷�Ӧ�����ӷ���ʽ��_________________________________________________��
 ��c(OH��)��0.1 mol��L��1������Һ��pH��13��(5)Na2O2����Һ���ܽ�Fe(OH)2����ΪFe(OH)3������ͬʱ��
��c(OH��)��0.1 mol��L��1������Һ��pH��13��(5)Na2O2����Һ���ܽ�Fe(OH)2����ΪFe(OH)3������ͬʱ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��ŷ�ӦFe(s)+CO2(g) FeO(s)+CO(g) ��H1��ƽ�ⳣ��ΪK1��
FeO(s)+CO(g) ��H1��ƽ�ⳣ��ΪK1��
��ӦFe(s)+H2O(g) FeO(s)+H2(g) ��H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)+H2(g) ��H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
| 700�� | 900�� | |
| K1 | 1��47 | 2��15 |
| K2 | 2��38 | 1��67 |
�ٷ�ӦCO2(g) + H2(g) CO(g) + H2O(g) ��H��ƽ�ⳣ��ΪK�����H= ���á�H1�͡�H2��ʾ����K= ����K1��K2��ʾ�����������������֪����ӦCO2(g) + H2(g)
CO(g) + H2O(g) ��H��ƽ�ⳣ��ΪK�����H= ���á�H1�͡�H2��ʾ����K= ����K1��K2��ʾ�����������������֪����ӦCO2(g) + H2(g) CO(g) + H2O(g)�� ��Ӧ������ȡ����ȡ�����
CO(g) + H2O(g)�� ��Ӧ������ȡ����ȡ�����
�����ж�CO2(g) + H2(g) CO(g) + H2O(g)�ﵽ��ѧƽ��״̬�������� ������ţ���
CO(g) + H2O(g)�ﵽ��ѧƽ��״̬�������� ������ţ���
A��������ѹǿ���� B�����������c(CO)����
C��v��(H2)= v��(H2O) D��c(CO)= c(CO2)
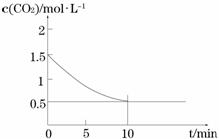 ��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)+CO2(g)
��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)+CO2(g) FeO(s)+CO(g) ��H > 0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)+CO(g) ��H > 0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
�� �������·�Ӧ��ƽ�ⳣ��Ϊ ��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ_________mol��L��1��
�����д�ʩ����ʹƽ��ʱ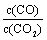 �������______������ţ���
�������______������ţ���
A�������¶� B������ѹǿ
C������һ������CO2 D���ټ���һ��������
 2Fe2+��I2��Ƴ�����ͼ��ʾ��ԭ��ء������жϲ���ȷ���ǣ� ��
2Fe2+��I2��Ƴ�����ͼ��ʾ��ԭ��ء������жϲ���ȷ���ǣ� ��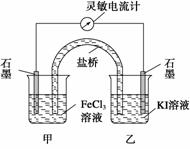
 ֪D��E��Z����ѧ��ѧ�����ĵ��ʣ��������ǻ����Z��Y���ȼҵ�IJ�Ʒ��DԪ
֪D��E��Z����ѧ��ѧ�����ĵ��ʣ��������ǻ����Z��Y���ȼҵ�IJ�Ʒ��DԪ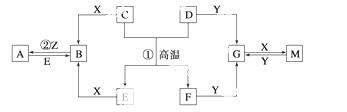
 _________��G___________________��Y______________��
_________��G___________________��Y______________��