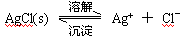��Ŀ����
��16�֣��״������͵���������ȼ�ϡ���ҵ�Ͽ�ͨ��H2��CO�����Ʊ��״����÷�Ӧ���Ȼ�ѧ����ʽΪ��2H2(g)��CO(g) CH3OH(g)
CH3OH(g) 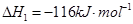
��1����֪��
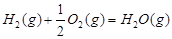
1 mol�״�������ȫȼ������CO ��ˮ�������Ȼ�ѧ����ʽΪ ��
��ˮ�������Ȼ�ѧ����ʽΪ ��
��2�����д�ʩ�����������2H2(g)��CO(g) CH3OH(g)��Ӧ���ʵ��� ��˫ѡ)��
CH3OH(g)��Ӧ���ʵ��� ��˫ѡ)��
��3����3����H2��CO�����Ʊ��״��ķ�Ӧ�У�����Ӧ���ݻ�Ϊ1L�ĺ����������ֱ���230�桢250���270���£��ı�H2��CO����ʼ��ɱȣ���ʼʱCO�����ʵ����̶�Ϊ1mol������ʵ�飬�������ͼ��ʾ��ͼ�������ϵĵ㶼Ϊһ���¶��¡�һ������µ�ƽ��㣩��
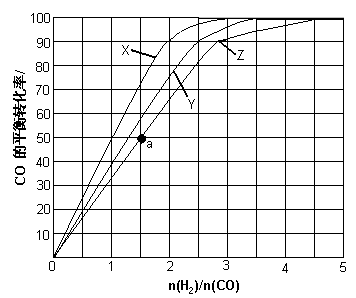
������X��Ӧ���¶��� ��
�ڴ�ͼ�п��Եó��Ľ����� ����дһ������
��4��������Ӧ���ݻ��м���1.5molH2��1.0molCO��������Z��Ӧ�¶��·�Ӧ��ƽ�⡣��������ͼ��a���Ӧ��COƽ��ת���ʣ�����2H2(g)��CO(g) CH3OH(g)��ƽ�ⳣ������д��������̣�
CH3OH(g)��ƽ�ⳣ������д��������̣�
 CH3OH(g)
CH3OH(g) 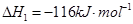
��1����֪��
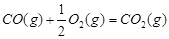
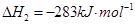
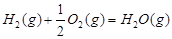
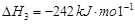
1 mol�״�������ȫȼ������CO
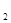 ��ˮ�������Ȼ�ѧ����ʽΪ ��
��ˮ�������Ȼ�ѧ����ʽΪ ����2�����д�ʩ�����������2H2(g)��CO(g)
 CH3OH(g)��Ӧ���ʵ��� ��˫ѡ)��
CH3OH(g)��Ӧ���ʵ��� ��˫ѡ)��| A�������CH3OH | B�������¶� | C����Сѹǿ | D��������ʵĴ��� |

������X��Ӧ���¶��� ��
�ڴ�ͼ�п��Եó��Ľ����� ����дһ������
��4��������Ӧ���ݻ��м���1.5molH2��1.0molCO��������Z��Ӧ�¶��·�Ӧ��ƽ�⡣��������ͼ��a���Ӧ��COƽ��ת���ʣ�����2H2(g)��CO(g)
 CH3OH(g)��ƽ�ⳣ������д��������̣�
CH3OH(g)��ƽ�ⳣ������д��������̣�(1) 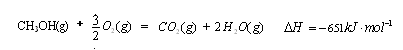 (3��)
(3��)
��2��BD ��4�֣� ��3����230�棨3�֣�
�������������䣬CO��ƽ��ת��������H2��CO����ʼ��ɱ���������������������䣬CO��ƽ��ת�������¶����߶����͡��� ��2�֣�
��4��a��H2��CO����ʼ��ɱ�Ϊ1.5��CO��ƽ��ת����Ϊ50������1�֣�
2H2(g)��CO(g) CH3OH(g)
CH3OH(g)
��ʼ���ʵ���(mol) 1.5 1 0
ת�����ʵ���(mol�� 1 0.5 0.5
ƽ�����ʵ���(mol) 0.5 0.5 0.5
ƽ��Ũ��(mol/L) 0.5 0.5 0.5 ��1�֣�
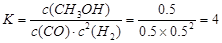 ��2�֣�
��2�֣�
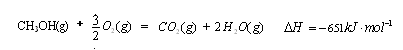 (3��)
(3��)��2��BD ��4�֣� ��3����230�棨3�֣�
�������������䣬CO��ƽ��ת��������H2��CO����ʼ��ɱ���������������������䣬CO��ƽ��ת�������¶����߶����͡��� ��2�֣�
��4��a��H2��CO����ʼ��ɱ�Ϊ1.5��CO��ƽ��ת����Ϊ50������1�֣�
2H2(g)��CO(g)
 CH3OH(g)
CH3OH(g)��ʼ���ʵ���(mol) 1.5 1 0
ת�����ʵ���(mol�� 1 0.5 0.5
ƽ�����ʵ���(mol) 0.5 0.5 0.5
ƽ��Ũ��(mol/L) 0.5 0.5 0.5 ��1�֣�
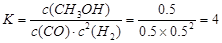 ��2�֣�
��2�֣���1�������˹���ɵ�Ӧ�á�������֪��Ӧ��֪���ڣ��ۡ�2���ټ��õ�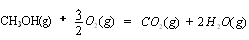 �����Է�Ӧ���ǣ�283kJ/mol��242 kJ/mol��2��116 kJ/mol����651 kJ/mol.
�����Է�Ӧ���ǣ�283kJ/mol��242 kJ/mol��2��116 kJ/mol����651 kJ/mol.
��2��������������Է�Ӧ���ʶ�Ӱ�졣����Ӧ���Ũ�Ȼ������¶Ȼ�ʹ�ô����ȶ����Լӿ췴Ӧ���ʣ�BD��ȷ��AC���ǽ��ͷ�Ӧ���ʵģ���ѡBD��
��3����Ӧ�÷�Ӧ�Ƿ��ȷ�Ӧ�������¶�Խ�ߣ�ת����Խ�͡�X���߱�ʾ��ת������������¶���ͣ���X��ʾ����230�档
�ڸ���ͼ���֪�������������䣬CO��ƽ��ת��������H2��CO����ʼ��ɱ����������
��4������ƽ�ⳣ�����йؼ��㡣
a��H2��CO����ʼ��ɱ�Ϊ1.5��CO��ƽ��ת����Ϊ50��
2H2(g)��CO(g) CH3OH(g)
CH3OH(g)
��ʼ���ʵ���(mol) 1.5 1 0
ת�����ʵ���(mol�� 1 0.5 0.5
ƽ�����ʵ���(mol) 0.5 0.5 0.5
ƽ��Ũ��(mol/L) 0.5 0.5 0.5
����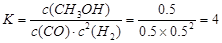
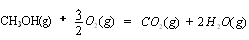 �����Է�Ӧ���ǣ�283kJ/mol��242 kJ/mol��2��116 kJ/mol����651 kJ/mol.
�����Է�Ӧ���ǣ�283kJ/mol��242 kJ/mol��2��116 kJ/mol����651 kJ/mol.��2��������������Է�Ӧ���ʶ�Ӱ�졣����Ӧ���Ũ�Ȼ������¶Ȼ�ʹ�ô����ȶ����Լӿ췴Ӧ���ʣ�BD��ȷ��AC���ǽ��ͷ�Ӧ���ʵģ���ѡBD��
��3����Ӧ�÷�Ӧ�Ƿ��ȷ�Ӧ�������¶�Խ�ߣ�ת����Խ�͡�X���߱�ʾ��ת������������¶���ͣ���X��ʾ����230�档
�ڸ���ͼ���֪�������������䣬CO��ƽ��ת��������H2��CO����ʼ��ɱ����������
��4������ƽ�ⳣ�����йؼ��㡣
a��H2��CO����ʼ��ɱ�Ϊ1.5��CO��ƽ��ת����Ϊ50��
2H2(g)��CO(g)
 CH3OH(g)
CH3OH(g)��ʼ���ʵ���(mol) 1.5 1 0
ת�����ʵ���(mol�� 1 0.5 0.5
ƽ�����ʵ���(mol) 0.5 0.5 0.5
ƽ��Ũ��(mol/L) 0.5 0.5 0.5
����
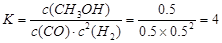

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ