��Ŀ����
������ͼ�ش�����
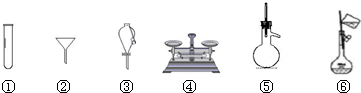
��1��д����ͼ�����Ϊ�١������������ƣ�
��______����______����______����______��
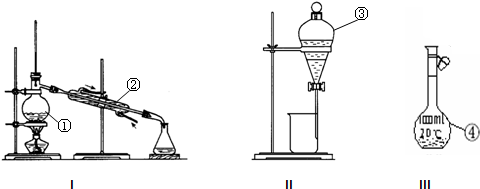
��2�������ˮ�еĵ�Ӧ��ѡ��װ��______����װ����ţ�����______��______����������װ��______����װ����ţ�����______������
��3����������������Ϊ98%���ܶ�Ϊ1.84g?cm-3��Ũ����������Ũ��Ϊ0.50mol?L-1��ϡ����100mL��
����ȡŨ��������Ϊ______mL��
�����в��������������Ƶ���ҺŨ��ƫ�ߵ���______
A����ȡŨ����ʱ����B�����ձ���ϡ��Ũ���������ת�Ƶ�����ƿ��
C������ʱ����D��ϴ��������ƿδ�����������������Һ��

��1��д����ͼ�����Ϊ�١������������ƣ�
��______����______����______����______��
��2�������ˮ�еĵ�Ӧ��ѡ��װ��______����װ����ţ�����______��______����������װ��______����װ����ţ�����______������
��3����������������Ϊ98%���ܶ�Ϊ1.84g?cm-3��Ũ����������Ũ��Ϊ0.50mol?L-1��ϡ����100mL��
����ȡŨ��������Ϊ______mL��
�����в��������������Ƶ���ҺŨ��ƫ�ߵ���______
A����ȡŨ����ʱ����B�����ձ���ϡ��Ũ���������ת�Ƶ�����ƿ��
C������ʱ����D��ϴ��������ƿδ�����������������Һ��
��1����١������������Ʒֱ�Ϊ��������ƿ�������ܡ���Һ©����100mL����ƿ��
�ʴ�Ϊ��������ƿ�������ܣ���Һ©����100mL����ƿ��
��2���������Ȼ�̼�е��ܽ�Ƚ���ˮ�д������Ȼ�̼��ˮ�������ܣ�������ȡ�ķ������룬���ſ��÷�Һ����������Һ��ֿ������Ȼ�̼�ӷ�����������ķ�������õ����Ȼ�̼��
�ʴ�Ϊ������ȡ����Һ��������
��3��������Ҫ98%H2SO4�����ΪVmL��������Һϡ��ǰ����������������VmL��1.84g/cm3��98%=100mL��0.50mol?L-1��98g/mol�����V=27ml��������Һ�����Ϊ27ml��
�ʴ�Ϊ��27��
��A����ȡŨ����ʱ���ӻ�ʹ��Ũ��������ƫС������ʹ��ҺŨ��ƫ�ͣ�
B�����ձ���ϡ��Ũ����ʱ�ų��ȣ���������ת�Ƶ�����ƿ�У���ʹ����Һ��������ͣ��ȵ���ȴ�����£�ʹ����Һ�������С������ʹ��ҺŨ��ƫ�ߣ�
C������ʱ���ӣ�ʹ����Һ�������С������ʹ��ҺŨ��ƫ�ߣ�
D��ϴ��������ƿδ�����������������Һ������Ӱ����ҺŨ�ȣ�
�ʴ�Ϊ��BC��
�ʴ�Ϊ��������ƿ�������ܣ���Һ©����100mL����ƿ��
��2���������Ȼ�̼�е��ܽ�Ƚ���ˮ�д������Ȼ�̼��ˮ�������ܣ�������ȡ�ķ������룬���ſ��÷�Һ����������Һ��ֿ������Ȼ�̼�ӷ�����������ķ�������õ����Ȼ�̼��
�ʴ�Ϊ������ȡ����Һ��������
��3��������Ҫ98%H2SO4�����ΪVmL��������Һϡ��ǰ����������������VmL��1.84g/cm3��98%=100mL��0.50mol?L-1��98g/mol�����V=27ml��������Һ�����Ϊ27ml��
�ʴ�Ϊ��27��
��A����ȡŨ����ʱ���ӻ�ʹ��Ũ��������ƫС������ʹ��ҺŨ��ƫ�ͣ�
B�����ձ���ϡ��Ũ����ʱ�ų��ȣ���������ת�Ƶ�����ƿ�У���ʹ����Һ��������ͣ��ȵ���ȴ�����£�ʹ����Һ�������С������ʹ��ҺŨ��ƫ�ߣ�
C������ʱ���ӣ�ʹ����Һ�������С������ʹ��ҺŨ��ƫ�ߣ�
D��ϴ��������ƿδ�����������������Һ������Ӱ����ҺŨ�ȣ�
�ʴ�Ϊ��BC��

��ϰ��ϵ�д�
�����Ŀ