��Ŀ����
�ö��Ե缫���һ����������ͭ��Һ��ʵ��װ����ͼ�٣��������е�ʵ��������ͼ�ڣ��������ʾ��������ת�Ƶ��ӵ����ʵ������������ʾ�������в�����������������״������������˵����ȷ���ǣ�������


��������ͼ��֪��������������������bΪ������aΪ���������Ե缫���һ����������ͭ��Һ������2CuSO4+2H2O
2Cu+O2��+2H2SO4�����ͼ2��֪��ͨ��0.2mol����ʱ�������ͭ��Ȼ����������Һ������2H2O
2H2��+O2����P��Q��ʱ�ռ����Ļ������Ϊ�������������Դ������
| ||
| ||
����⣺��ͼ��֪��������������������bΪ������aΪ���������Ե缫���һ����������ͭ��Һ������2CuSO4+2H2O
2Cu+O2��+2H2SO4�����ͼ2��֪��ͨ��0.2mol����ʱ�������ͭ��Ȼ����������Һ������2H2O
2H2��+O2����
A��bΪ��������Һ�е����������ӷŵ磬���к�ɫ������������A����
B��aΪ�������ȷ���Cu2++2e-=Cu������2H++2e-=H2������B����
C����Q��ʱ�ռ����Ļ������Ϊ�������������ɵ��ˮ��Ӧ��֪0.2mol����ͨ��ʱ����0.1molH2��0.05molO2�����������ƽ��Ħ������Ϊ
=12g?mol-1����C��ȷ��
D��������������֪������0��P�α�ʾO2������仯������P��Q�α�ʾH2��O2������������仯����D����
��ѡC��
| ||
| ||
A��bΪ��������Һ�е����������ӷŵ磬���к�ɫ������������A����
B��aΪ�������ȷ���Cu2++2e-=Cu������2H++2e-=H2������B����
C����Q��ʱ�ռ����Ļ������Ϊ�������������ɵ��ˮ��Ӧ��֪0.2mol����ͨ��ʱ����0.1molH2��0.05molO2�����������ƽ��Ħ������Ϊ
| 0.1mol��2g/mol+0.05mol��32g/mol |
| 0.1mol+0.05mol |
D��������������֪������0��P�α�ʾO2������仯������P��Q�α�ʾH2��O2������������仯����D����
��ѡC��
���������⿼����ԭ������ȷͼ�������ת������������Ĺ�ϵ�����ӵķŵ�˳���ǽ����Ĺؼ�����Ϥ���ԭ�����ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
�ö��Ե缫���һ����������ͭ��Һ��ʵ��װ������ͼ�ף��������е�ʵ����������ͼ�ң��������ʾ��������ת�Ƶ��ӵ����ʵ������������ʾ�������в�����������������״������������˵���в���ȷ���ǣ�������
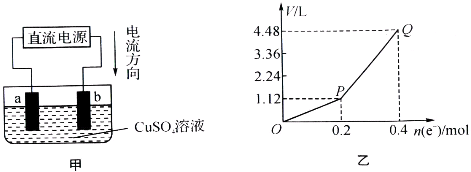

| A���������У�a�缫�������к�ɫ�����������������ݲ��� | ||||
B��b�缫�Ϸ����ķ�Ӧ����ʽΪ4OH--4e-
| ||||
| C������O��P�α�ʾH2������仯 | ||||
| D��Q��ʱ�ռ����Ļ��������H2��O2�����Ϊ1��1 |
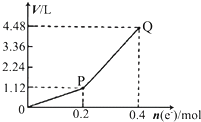 �ö��Ե缫���һ����������ͭ��Һ���������е�ʵ��������ͼ��ʾ���������ʾת�Ƶ��ӵ����ʵ������������ʾ�������в�����������������״�����������ж���ȷ���ǣ�������
�ö��Ե缫���һ����������ͭ��Һ���������е�ʵ��������ͼ��ʾ���������ʾת�Ƶ��ӵ����ʵ������������ʾ�������в�����������������״�����������ж���ȷ���ǣ�������