��Ŀ����
X��Y��Z���ֳ���Ԫ�صĵ��ʣ��ס��������ֳ����Ļ�����������ͼת����ϵ���ش��������⣺

��1�� ��X��̬ԭ����Χ�����Ų�ʽΪ3s2�������ɵڶ���������Ԫ�ص�ԭ�ӹ��ɵ���̬��������ӣ� Y��̬ԭ�ӵĹ����ʾʽΪ ���ĵ���ʽΪ
��2����Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����������YΪ�ӷ���Һ�����ʡ���Ϊ��ɫ������ˮ�����塣��ZΪ �����Y��Ԫ�صĻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
��3����X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����Ϊ���д��Եĺ�ɫ���壬��X���Ӧ�Ļ�ѧ����ʽΪ�� ��Y2+���ӵĵ����Ų�ʽΪ ��

��1�� ��X��̬ԭ����Χ�����Ų�ʽΪ3s2�������ɵڶ���������Ԫ�ص�ԭ�ӹ��ɵ���̬��������ӣ� Y��̬ԭ�ӵĹ����ʾʽΪ ���ĵ���ʽΪ
��2����Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����������YΪ�ӷ���Һ�����ʡ���Ϊ��ɫ������ˮ�����塣��ZΪ �����Y��Ԫ�صĻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
��3����X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����Ϊ���д��Եĺ�ɫ���壬��X���Ӧ�Ļ�ѧ����ʽΪ�� ��Y2+���ӵĵ����Ų�ʽΪ ��
��1��1s2 2s2
2s2 2p2
2p2 CO2�ĵ���ʽ
CO2�ĵ���ʽ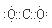
��2��H2��[Ar]3d104s24p5
��3��8Al+3Fe3O4 9Fe+ 4Al2O3 [Ar]3d6
9Fe+ 4Al2O3 [Ar]3d6
 2s2
2s2 2p2
2p2 CO2�ĵ���ʽ
CO2�ĵ���ʽ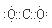
��2��H2��[Ar]3d104s24p5
��3��8Al+3Fe3O4
 9Fe+ 4Al2O3 [Ar]3d6
9Fe+ 4Al2O3 [Ar]3d6�����������1��X��̬ԭ����Χ�����Ų�ʽΪ3s2����X�ĺ��������Ϊ12������XΪþ����þ��Ӧ����̬������ӦΪ������̼�����Ϊ������̼��YΪ̼���ʡ�����Y�Ļ�̬ԭ�ӵĹ����ʾʽΪ1s2
 2s2
2s2 2p2
2p2 ,������̼�ĵ���ʽΪ
,������̼�ĵ���ʽΪ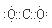
��2��Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2��,��n-1=2��n=3��X�����������Ų�Ϊ3s23p5��XΪ������������YΪ�ӷ���Һ�����ʣ���YΪҺ�壻��������Ϊ��ɫ������ˮ�����壬��Ϊ�Ȼ��⣬ZΪ����������35��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s24p5
��3��X��̬ԭ����Χ�����Ų�ʽΪ3s23p1��XΪ�����ʣ���Ϊ���д��Եĺ�ɫ���壬��Ϊ������������X���Ӧ�����ȷ�Ӧ����ѧ����ʽΪ8Al+3Fe3O4
 9Fe +4Al2O3 ��YΪ�����ʣ�Fe2+�ĵ����Ų�ʽΪ[Ar]3d6
9Fe +4Al2O3 ��YΪ�����ʣ�Fe2+�ĵ����Ų�ʽΪ[Ar]3d6
��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д�
�����Ŀ

