��Ŀ����
��֪A��B��C��D��EΪ���ֶ�����Ԫ�ع��ɵ����ӣ�A��B��C��D����10e-��EΪ˫��18e-���ӣ��ش��������⣺
��1��A��B�γɵĻ�������м������Ӽ����й��ۼ���A��C��ϳɵĻ�������������ˮ�������ڼ�ˮ��Һ����ɫ����Һ����C���ӵĽṹʾ��ͼΪ ____���÷�Ӧ�����ӷ���ʽΪ____ ____��
��2���κ�ˮ��Һ�ж�����A��D�������ӣ�D���ӵĵ���ʽΪ ____��
��3��B��E�γɵĻ������������������֮��Ϊ2��1��A��D��ϳɻ����ﶡ���������Ӧ������ɫ��ζ���壬д�����ĵ���ʽ ____ ���÷�Ӧ�Ļ�ѧ����ʽΪ��
____��
��4����һ��Һ̬���⻯�����죬�ǡ������ߺš��ɴ�����ʱʹ�õĸ���ȼ��֮һ���û�������E�ĵ�������ͬ���ṹ�����õ��÷�����ֻ���е���������ĵ���ʽΪ ____��
���𰸡�
��1�� ��1�֣���Al(OH)3
+ OH- = AlO2- + 2H2O��2�֣�
��1�֣���Al(OH)3
+ OH- = AlO2- + 2H2O��2�֣�
��2��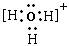 ��2�֣�
��2�֣�
��3�� ��2�֣���Na2O2 + CO2
= 4 NaOH + O2�� ��2�֣�
��2�֣���Na2O2 + CO2
= 4 NaOH + O2�� ��2�֣�
��4��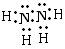 ��2�֣�
��2�֣�

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�