��Ŀ����
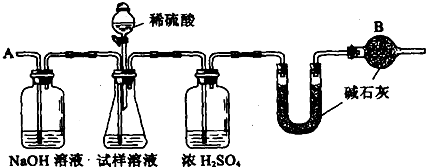
28.��֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣
��Ҫʵ�鲽�����£�
�ٰ�ͼ��װ�����������װ�õ�������
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�b g
�ܴӷ�Һ©������6mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ
�ݴӵ���A����������һ�����Ŀ���
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c g
���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊd g
����պͻش����⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��
��2��װ���и����B��������
��3���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ�� (��ƫ�ߡ�ƫ�ͻ�)
��4������ݵ�Ŀ����
��5������ߵ�Ŀ����
��6���������д����������������ʽΪ
��7��������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ��ʵ�鷽����
�𰸣�
��1����Ʒ�أ�������
��2����ֹ�����е�CO2��ˮ������U����
��3��ƫ��
��4���ѷ�Ӧ������CO2ȫ������U����
��5���жϷ�Ӧ������CO2�Ƿ�ȫ���ų�������U���еļ�ʯ������
��6��![]() ��100��
��100��
��7�����ԡ�
��������1��������ƽ����ʱ�����̷�ҩƷ�����̷����룬��ָ����ƫ��˵�����̽��أ�Ҳ����ҩƷ��������������������2��ͨ������ʵ�鿪ʼ�ͽ���ʱU�ιܵ�����������ȷ������CO2����������U�ιܺ��һ������ܣ����Է�ֹ�����е�CO2��ˮ�����Ƚ���U�ιܣ�ʹʵ������Ϊȷ����3���������ǻӷ����ᣬ���ɵ�CO2�л����HCl�������ܱ�ŨH2SO4��ȥ�������Ա���ʯ�����գ�Ҳ����ʵ��������ʯ�ҵ�������ƫ�����Ľ����ƫ�ߡ���4��CO2���ܶȽϴ����ɵ�CO2���ܻ��������ƿ�У���������������ɵ�CO2ȫ������U�ι��С���5��������γ���U�ιܵĽ���������䣬˵����ƿ�����е�CO2�Ķ�����ʯ�������ˡ�
��6��Na2CO3+H2SO4====Na2SO4+H2O+CO2��
106 g 44 g
m��Na2CO3�� ��d��b�� g
���ԣ�Na2CO3����������Ϊ��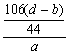 ��100%=
��100%=![]() ��100%
��100%
��7����ʵ��ķ����϶࣬������Գ���һ����������Ʒ������������ϡHNO3ʹCO2ȫ���ų���μ�����AgNO3������ϴ��������������ͨ������Ҳ��ȷ����Ʒ�Ĵ��ȡ���������ֻҪԭ����ȷ���������ж��ǿ��Եġ�

 �߽�������ϵ�д�
�߽�������ϵ�д�