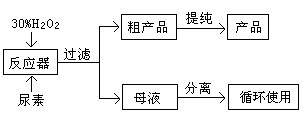
��Ŀ����
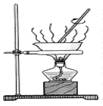
���ù�������KxFe��C2O4��y��3H2O��FeΪ��3�ۣ���ʵ�����Ʊ��Ͳⶨ����ɵķ���������ʾ��
��.�Ʊ���

��1����K2C2O4��FeCl3�Ʊ��������ϵķ�Ӧ����________������ţ���
�����ӷ�Ӧ���ڷ�������ԭ��Ӧ����������ԭ��Ӧ���ܻ��Ϸ�Ӧ
��2���ᾧʱӦ��������Һ���ںڰ����ȴ����������������������ԭ����_________��
��3������4��ʵ�������____________��
��.��ɲⶨ��
��ȡһ������ʵ�����õľ���������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0.10 mol��L��1 KMnO4��Һ���еζ�������KMnO4��Һ24.00 mLʱǡ����ȫ��Ӧ������������MnO4-�Ļ�ԭ������Mn2�������ټ��������Ļ�ԭ������Fe3����ȫת��ΪFe2������KMnO4��Һ�����ζ�����Fe2����ȫ����ʱ����ȥKMnO4��Һ4.00 mL��
��4�������������H2C2O4��Fe2�������ӷ���ʽ�ֱ���___________�� ________��
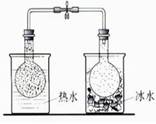
��5������100 mL 0.10 mol��L��1 KMnO4��Һ���������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ�⣬����________��________��д���ƣ���
��6��ͨ�����㣬�û�����Ļ�ѧʽ��____________��
��.�Ʊ���

��1����K2C2O4��FeCl3�Ʊ��������ϵķ�Ӧ����________������ţ���
�����ӷ�Ӧ���ڷ�������ԭ��Ӧ����������ԭ��Ӧ���ܻ��Ϸ�Ӧ
��2���ᾧʱӦ��������Һ���ںڰ����ȴ����������������������ԭ����_________��
��3������4��ʵ�������____________��
��.��ɲⶨ��
��ȡһ������ʵ�����õľ���������ƿ�У�����������ˮ��ϡH2SO4����C2O42-��ȫת��ΪH2C2O4����0.10 mol��L��1 KMnO4��Һ���еζ�������KMnO4��Һ24.00 mLʱǡ����ȫ��Ӧ������������MnO4-�Ļ�ԭ������Mn2�������ټ��������Ļ�ԭ������Fe3����ȫת��ΪFe2������KMnO4��Һ�����ζ�����Fe2����ȫ����ʱ����ȥKMnO4��Һ4.00 mL��
��4�������������H2C2O4��Fe2�������ӷ���ʽ�ֱ���___________�� ________��
��5������100 mL 0.10 mol��L��1 KMnO4��Һ���������ζ�ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ����ƿ�⣬����________��________��д���ƣ���
��6��ͨ�����㣬�û�����Ļ�ѧʽ��____________��
��.��1���٢�
��2����ֹ�������ֽ�
��3�����ˡ�ϴ�ӡ�����
��.��4��2MnO42-��5H2C2O4��6H��=2Mn2����10CO2����8H2O��MnO4-��5Fe2����8H��=Mn2����5Fe3����4H2O
��5��100 mL����ƿ����ʽ�ζ���
��6��K3Fe��C2O4��3��3H2O
��2����ֹ�������ֽ�
��3�����ˡ�ϴ�ӡ�����
��.��4��2MnO42-��5H2C2O4��6H��=2Mn2����10CO2����8H2O��MnO4-��5Fe2����8H��=Mn2����5Fe3����4H2O
��5��100 mL����ƿ����ʽ�ζ���
��6��K3Fe��C2O4��3��3H2O
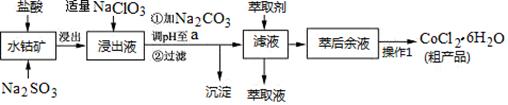
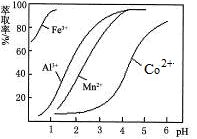
��.��1����Ϊ��������KxFe��C2O4��y��3H2O�е�FeΪ��3�ۣ�����Ӧǰ��û�л��ϼ۱仯�������ֵ��������Һ�н��еķ�Ӧ���ڷ�������ԭ��Ӧ�����ӷ�Ӧ����2�����ڸò����ǹ������ϣ������ֽ⣬���ԽᾧʱӦ�ںڰ������С���3���ؽᾧ����뾭�����ˡ�ϴ�Ӻ�����ܻ�ô����ľ��塣
��.��4�����ݲ�����C�Ļ��ϼ��ǣ�3��������������������������ֻ����CO2��Fe2������������ֻ����Fe3������5��������Һ����Ҫ100 mL����ƿ���ζ�������Ҫ�ζ��ܣ������������Һ��ǿ�����ԣ��������ܣ�����Ҫ��ʽ�ζ��ܡ�
��6�����ݵ����غ����ݷ���ʽ��n��C2O42-����2.5��0.10 mol��L��1��0.024 L��0.006 mol��n��Fe3������5��0.10 mol��L��1��0.004 L��0.002 mol���ٸ��ݻ��ϼ۴�����Ϊ�㣬��ѧʽ��K3Fe��C2O4��3��3H2O��
��.��4�����ݲ�����C�Ļ��ϼ��ǣ�3��������������������������ֻ����CO2��Fe2������������ֻ����Fe3������5��������Һ����Ҫ100 mL����ƿ���ζ�������Ҫ�ζ��ܣ������������Һ��ǿ�����ԣ��������ܣ�����Ҫ��ʽ�ζ��ܡ�
��6�����ݵ����غ����ݷ���ʽ��n��C2O42-����2.5��0.10 mol��L��1��0.024 L��0.006 mol��n��Fe3������5��0.10 mol��L��1��0.004 L��0.002 mol���ٸ��ݻ��ϼ۴�����Ϊ�㣬��ѧʽ��K3Fe��C2O4��3��3H2O��

��ϰ��ϵ�д�
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
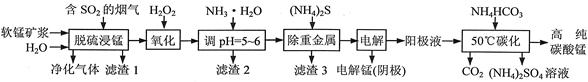
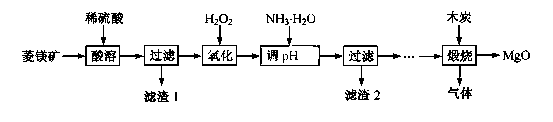
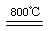 2MgO+2SO2��+CO2��
2MgO+2SO2��+CO2��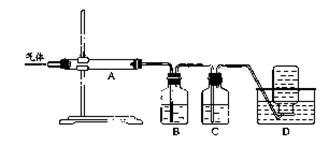
 N2O4��g�� ��H<0
N2O4��g�� ��H<0