��Ŀ����
��1���ڹ̶�������ܱ�������ͨ��N2��H2��������˵���ﵽƽ�����________��
| A��3v��N2����v��H2�� |
| B������1��N��N��ͬʱ����6��N��H |
| C��N2��H2��NH3�����ʵ���֮����1��3��2 |
| D�������������ѹǿ���� |
F�������ƽ����Է�����������
��2����2 L���ܱ�������ͨ��2 mol N2��8 mol H2��5 minʱ�ﵽƽ�⣬���NH3�����ʵ�����2 mol����ƽ��ʱc��H2����______________��
��1��BDF����2��2.5 mol/L
����

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д���һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У�������Ũ����ʱ��仯�Ĺ�ϵ��ͼ1��ʾ��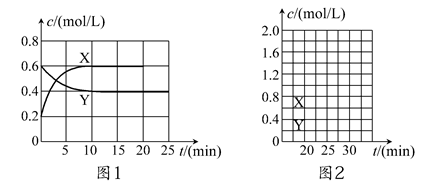
��ش�
(1)ͼl�У�����________(�X����Y��)��ʾNO2Ũ����ʱ��ı仯�����ǰ10 min��v(NO2)��________mol/(L��min)��
(2)����ѡ���в���˵���÷�Ӧ�Ѵﵽƽ��״̬����________(��ѡ����ĸ)��
| A�������ڻ�������ѹǿ����ʱ��仯���ı� |
| B�������ڻ��������ܶȲ���ʱ��仯���ı� |
| C�������ڻ���������ɫ����ʱ��仯���ı� |
| D�������ڻ�������ƽ����Է�����������ʱ��仯���仯 |
(4)��Ӧ���е�20 minʱ�����������ڳ���һ����NO2,10 min��ﵽ�µ�ƽ�⣬��ʱ���c(NO2)��0.9 mol/L��
�ٵ�һ��ƽ��ʱ���������NO2���������Ϊ��1���ﵽ��ƽ�����������NO2���������Ϊ��2�����1________��2(�>������������<��)��
������ͼ2�л���20 min������ʵ�Ũ����ʱ��仯������(�����ϱ�������X���͡�Y��)��
������Ҫ�ĵ��ʣ��Dz����ϴ�Ļ�����Ʒ֮һ���α�����ܵĺϳɰ�������Ϊ���������ǵ¹��˹�����1905�귢���ģ���ϳ�ԭ��Ϊ��
N2(g)��3H2(g) 2NH3(g) ��H����92.4 kJ��mol��1
2NH3(g) ��H����92.4 kJ��mol��1
����˻����1918��ŵ������ѧ�����Իش��������⣺
(1)�ϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵���________��
| A�����ýϸ�ѹǿ |
| B������500 ��ĸ��� |
| C��������ý������ |
| D�������ɵİ�Һ������ʱ����ϵ�з��������ʣ��N2��H2ѭ�����ϳ��� |
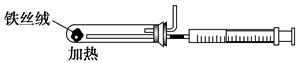
(2)��ͼ��ʵ����ģ�ҵ�ϳɰ��ļ���װ�ã����������а������ɵķ�����
_________________________________________________________________��
(3)��298 Kʱ����10 mol N2��30 mol H2ͨ��ϳ����У��ų�������С��924kJ��ԭ����______________________________
(4)1998��ϣ������˹��´�ѧ��Marmellos��Stoukides���ø����ӵ�����
��SCY�մ�(�ܴ���H��)��ʵ���˸��¡���ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����
ʵ��װ������ͼ�����������ĵ缫��ӦʽΪ____________________________��

��ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
��1�����á���ѧ����ת�Ʒ����ᴿ�����ٵķ�Ӧԭ��ΪW(s)��I2(g) WI2(g) (I)���÷�Ӧ��ʯӢ��չ��н��У�����ͼ��ʾ��
WI2(g) (I)���÷�Ӧ��ʯӢ��չ��н��У�����ͼ��ʾ��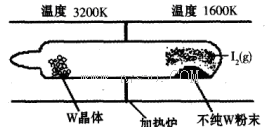
�ٷ�Ӧ(I)��ƽ�ⳣ������ʽK��_______����K��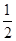 ����ij�����ܱ������м���1mol I2(g)������W(s)����Ӧ�ﵽƽ��ʱI2(g)��ת����Ϊ__________��
����ij�����ܱ������м���1mol I2(g)������W(s)����Ӧ�ﵽƽ��ʱI2(g)��ת����Ϊ__________��
�ڷ�Ӧ(I)�ġ�H____0���������������������Ӧ��ϵ�п�ѭ��ʹ�õ�������_________��
���ܹ�˵��������Ӧ�Ѿ��ﵽƽ��״̬����_________������ţ���
a��I2��WI2��Ũ�����
b��W���������ٱ仯
c�������ڻ��������ܶȱ��ֲ���
d����λʱ���ڣ����������ĵ����ʵ�����⻯�����ɵ����ʵ������
��2��25��ʱ��NaHSO3��ˮ��ƽ�ⳣ����1.0��10��12mol/L������¶���H2SO3 HSO3����H���ĵ��볣��Ka��____mol/L������H2SO3��Һ�м���������I2������Һ��
HSO3����H���ĵ��볣��Ka��____mol/L������H2SO3��Һ�м���������I2������Һ��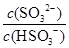 ��________�����������С�����䡱����
��________�����������С�����䡱����
��3��ֱ���ŷź�SO2���������γ����꣬Σ����������Na2SO3��Һ����SO2�Ĺ����У�pH��n��SO32������n(HSO3��)�仯��ϵ���±���
| n(SO32��): n ( HSO3��) | 91:9 | 1:1 | 1:91 |
| pH(25��) | 8.2 | 7.2 | 6.2 |
������Һ������ʱ����Һ������Ũ���ɴ�С��˳��Ϊ_________________________��
 C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc(A)��0.100 mol/L��c(B)��0.200 mol/L��c(C)��0 mol/L����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��
C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc(A)��0.100 mol/L��c(B)��0.200 mol/L��c(C)��0 mol/L����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��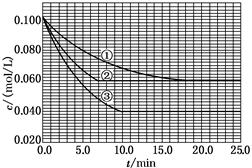
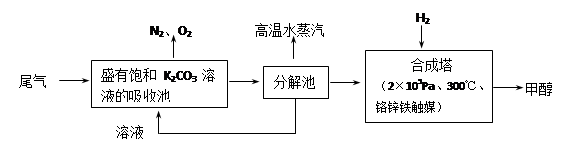
 CH3OH(g)��H2O(g)�����ܱ������£�����ʾ��ͼ��˵����Ӧ���е�t1ʱ��ʱ�ﵽƽ��״̬���� (����ĸ���)
CH3OH(g)��H2O(g)�����ܱ������£�����ʾ��ͼ��˵����Ӧ���е�t1ʱ��ʱ�ﵽƽ��״̬���� (����ĸ���)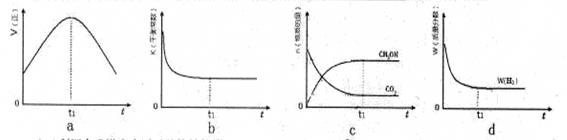
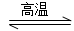 Si3N4(s)��12HCl(g)����H<0
Si3N4(s)��12HCl(g)����H<0

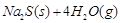 ����ش��������⣺
����ش��������⣺