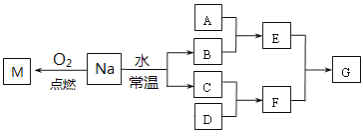
��Ŀ����
����Ŀ��������������־������־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ___________��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ_______��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����___________������Ϊ�ȵ�����ķ�����___________
����[Ni(NH3)6]SO4��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ______���ṩ�µ��ӶԵijɼ�ԭ����______��
��3������ͭ����������____���γɵľ��壻Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1 958 kJ��mol�C1��INi=1 753 kJ��mol�C1��ICu> INi��ԭ����______________________��
��4��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��

�پ�����ͭԭ������ԭ�ӵ�������Ϊ____________��
�����Ͻ���ܶ�Ϊd g��cm�C3����������a=__________nm��
��5��������ͭ��Һ�м��������ˮ�������ɣ�Cu(NH3)4��2+ �����ӡ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3��NH3���Dz�ͬ�����м��ǽϴ����____________����ԭ����_______________________����NF3������Cu2+�γ������ӣ���ԭ���ǣ�_______________
���𰸡� [Ar]3d84s2 2�� �������� CCl4 ��λ�� N ���� ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���� 3��1 ![]() NH3 ��ԭ�Ӻ��н϶�ŵ��Ӷ� �ܵ���ԭ�ӵ�һ�Թµ��ӶԵij����ϴ� �� NF3�ļ��DZ�NH3С �縺�Դ�С˳��ΪF �� N �� H���� NF3 �У����õ��Ӷ�ƫ�� F ԭ�ӣ�ƫ�� N ԭ�ӣ�ʹ�õ�ԭ���ϵŵ��Ӷ����� Cu2���γ���λ��
NH3 ��ԭ�Ӻ��н϶�ŵ��Ӷ� �ܵ���ԭ�ӵ�һ�Թµ��ӶԵij����ϴ� �� NF3�ļ��DZ�NH3С �縺�Դ�С˳��ΪF �� N �� H���� NF3 �У����õ��Ӷ�ƫ�� F ԭ�ӣ�ƫ�� N ԭ�ӣ�ʹ�õ�ԭ���ϵŵ��Ӷ����� Cu2���γ���λ��
����������1��1s22s22p63s23p63d84s2��[Ar]3d84s2��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ2��
��2����SO42-������ԭ��S��Χ��4���Ҽ������������������ͽṹ������5��ԭ�ӣ��۵�������Ϊ32�����以Ϊ�ȵ������������CCl4��
����λ������������ԭ�������������λ����Nԭ���ṩ�µ��Ӷԡ�
��3��������ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ�
��4����1�������к�Cuԭ�ӣ�6��1/2=3������Niԭ�ӣ�8��1/8=1�����ʾ�����Cu��Ni��ԭ�Ӹ�����Ϊ3:1��
��Cu3Ni��Ħ������Ϊ251g��mol-1����m=��V�ã�a3��dg��cm-3��NA=251g��mol-1�����![]() ��
��
��5��NH3����ԭ�Ӻ��н϶�ŵ��Ӷ� �ܵ���ԭ�ӵ�һ�Թµ��ӶԵij����ϴ� �� NF3�ļ��DZ�NH3С���縺�Դ�С˳��ΪF �� N �� H���� NF3 �У����õ��Ӷ�ƫ�� F ԭ�ӣ�ƫ�� N ԭ�ӣ�ʹ�õ�ԭ���ϵŵ��Ӷ����� Cu2���γ���λ����

 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�