��Ŀ����
����Ŀ��[��ѧ����ѡ��3�����ʽṹ������]
ԭ���������������A��B��C��D��E��F����Ԫ�ء�����A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��Cλ��ͬһ���壬F��ԭ������Ϊ29��
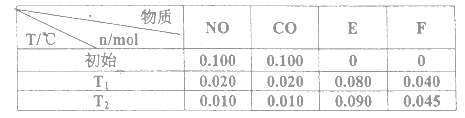
��1��F��̬ԭ�ӵĺ�������Ų�ʽΪ______________________��
��2����A��B��C����Ԫ���У���һ��������С�����˳����___________����Ԫ�ط��Żش𣩡�
��3��Ԫ��B�ļ���̬�⻯��ķе�___________������������������������Ԫ��A�ļ���̬�⻯��ķе㣬����Ҫԭ����______________________��
��4����A��B��C�γɵ�����CAB��AC2��Ϊ�ȵ����壬��CAB�ĽṹʽΪ___________��
��5����Ԫ��A��E���γɵij����������У�Aԭ�ӹ�����ӻ�����Ϊ___________��
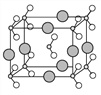
��6����B��C��D����Ԫ���γɵĻ����ᄃ��ľ�����ͼ��ʾ����û�����Ļ�ѧʽΪ___________��
��7��FC�ڼ�������������ת��ΪF2C����ԭ�ӽṹ�ĽǶȽ���ԭ��______________________��
���𰸡� 1s22s22p63s23p63d104s1��[Ar]3d104s1 C<O<N ���� NH3����֮������������CH4���Ӽ�������Ƿ��»���������ȷ��»�����ǿ [N=C=O] sp NaNO2 Cu+��Χ����3d10���ȫ�����ȶ���Cu2+��Χ����3d9������ӷ�ȫ����״̬���ȶ�
��������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��еĵ�������ȣ����A�ĺ�������Ų�ʽΪ1s22s22p2����AΪC��C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ����CΪO����BΪN��E��Cλ��ͬһ���壬��EΪS��DΪ����������ԭ�Ӱ뾶��������Ԫ�أ���DΪNa��F��ԭ������Ϊ29����FΪCu����1��FΪds������˺�������Ų�ʽΪ��1s22s22p63s23p63d104s1��[Ar]3d104s1����2��ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA����һ�����ܴ�С˳����N>O>C����3��B���⻯����NH3��A�ļ��⻯����CH4��NH3����֮������������CH4���Ӽ�������Ƿ��»���������ȷ��»�����ǿ����4��CAB���Ļ�ѧʽΪOCN����AC2�Ļ�ѧʽΪCO2������Ϊ�ȵ����壬���ǵĽṹ���ƣ����OCN���ĽṹʽΪ��[N=C=O] ����5���γɵĻ�������CS2���ṹʽΪS=C=S���ӻ���������ڼ۲���Ӷ�������C���ӻ�����Ϊsp����6�����ݰ뾶��С���������Na�������λ�ھ��������ϣ�����������ھ����ĸ���Ϊ8��1/4=2�������ΪN��С����ΪO�������γ�������NO2������λ�ڶ�������ģ������ĸ���Ϊ8��1/8=1=2����˻�ѧʽΪNaNO2����7��FCΪCuO��F2CΪCu2O��Cu�ĺ�������Ų�ʽ1s22s22p63s23p63d104s1��Cu2���ĵ����Ų�ʽΪ1s22s22p63s23p63d9��Cu���ĵ����Ų�ʽΪ1s22s22p63s23p63d10�����ݺ��ع����ڰ�����ȫ����ȫ�գ�����ȶ���ԭ����Cu+��Χ����3d10���ȫ�����ȶ���Cu2+��Χ����3d9������ӷ�ȫ����״̬���ȶ���

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�����Ŀ���±�ΪԪ�����ڱ���һ���֡�
̼ | �� | Y | |
X | �� | Z |
����˵������ȷ����
A. �ǽ����ԣ�Y����
B. ���Ӱ뾶��Y2-��S2-��Z-
C. ���������ԣ�Z���ʣ�����
D. ZԪ�������ڱ���λ�ڵ������ڵڢ�A��