��Ŀ����
��ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
����ͼװ����ʾ��A��B�еĵ缫Ϊ��Ķ��Ե缫��C��DΪ���ڽ���Na2SO4��Һ����ֽ���ϵIJ��У���Դ��a��b��������A��B�г���KOH��Һ������KOH��Һ��ˮ���С��ж�K1���պ�K2��K3ֱͨ���硣

�ش��������⣺
��1�� a�ǵ�Դ�� ����д��A�еĵ缫��ӦʽΪ_____��
��2��ʪ��Na2SO4��ֽ�����ܹ۲쵽��������____________��
��3�����һ��ʱ���A��B�о��������Χ�缫������ʱ�ж�K2��K3���պ�K1�����ֵ�������ָ���ƶ���д����ʱB�еĵ缫��ӦʽΪ ��
�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ҵ��һ��������з�Ӧ�ϳɼ״���CO(g)��2H2(g)
 CH3OH(g)
��H���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)����ش��������⣺
CH3OH(g)
��H���±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)����ش��������⣺
|
�¶� |
250�� |
300�� |
350�� |
|
K |
2.041 |
0.270 |
0.012 |
��4���ɱ��������жϦ�H 0�����������������������
��5�������������䣬ֻ�ı�����һ�����������д�ʩ����״����ʵ��� ��
A�������¶ȣ�B��ʹ�ú��ʵĴ�����C����С�������ݻ���D�����������H2��E����ѹʱ������He��F������ϵ�з����CH3OH
��6��ij�¶��£���2mol CO��6 mol H2����2L�ܱ������У���Ӧ���е�4minĩ�ﵽƽ�⣬��ʱ���c(CO) ��0.2 mol/L ����0~4min��H2�ķ�Ӧ����Ϊ ���������¶��ݻ������������г���һ������CH3OH�����´ﵽ��ѧƽ��״̬����ԭƽ��״̬��ȣ���ʱƽ����������CH3OH��������� ����������С�������䡱����
��1������2�֣���4OH�D�D4e�D=O2��+2H2O��2�֣�
��2����ɫ��D���ƶ���C��D���������������ݲ�����2�֣�
��3��H2�D2e�D+2OH�D=2H2O��2�֣�
��4������2�֣�
��5��C��D��F��2�֣���ѡ1����1�֣�����Ϊֹ��ѡ��1����0�֣�
��6��0.4mol/��L��min����2�֣���λ��1�֣������2�֣�
��������
�����������1���ж�K1���պ�K2��K3ֱͨ���磬���ˮ����KOH��Һ����������������������B����������Ϊ������A����������Ϊ������a�ǵ�Դ�ĸ�����д��A�еĵ缫��ӦʽΪ4OH�D�D4e�D=O2��+2H2O����2����ʪ��Na2SO4��ֽ���ϵ��ˮ��D���Դ����������Ϊ�����������Ӹ�����ظ�������D���ƶ��������ֱ������������������3�����һ��ʱ���A��B�о��������Χ�缫������ʱ�ж�K2��K3���պ�K1���γ�����ȼ�ϵ�أ�����Ϊ���ԣ���B�еĵ缫��ӦʽH2�D2e�D+2OH�D=2H2O��
��4���÷�Ӧ���¶ȸ�KֵС�����¶�����ƽ�������ƶ�����Ӧ���ȣ���5��A�������¶ȣ�ƽ�������ƶ�������B��ʹ�ú��ʵĴ�����ƽ�ⲻ�ƶ�������C����С�������ݻ�����ѹǿ��ƽ�������ƶ�����ȷ��D�����������H2 E����ѹʱ������He����Ӧ����ѹǿ���ͣ�ƽ�������ƶ�������F������ϵ�з����CH3OH��ƽ�������ƶ�����ȷ��
��6��0.4mol/��L��min�����������¶��ݻ������������г���һ������CH3OH������������ѹǿ���ƽ�������ƶ���CH3OH������������
���㣺���黯ѧ��Ӧԭ���е绯ѧ���Ȼ�ѧ����ѧƽ����й����⡣

 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�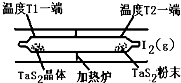
 ��2����ͼ��ʾ����Ӧ��I����ʯӢ��չ��н��У������¶�ΪT2��һ�˷���δ�ᴿ��TaS2��ĩ������I2��g����һ��ʱ������¶�ΪT1��һ�˵õ��˴�����TaS2���壬���¶�T1 T2���������������=������������Ӧ��ϵ��ѭ��ʹ�õ������� ��
��2����ͼ��ʾ����Ӧ��I����ʯӢ��չ��н��У������¶�ΪT2��һ�˷���δ�ᴿ��TaS2��ĩ������I2��g����һ��ʱ������¶�ΪT1��һ�˵õ��˴�����TaS2���壬���¶�T1 T2���������������=������������Ӧ��ϵ��ѭ��ʹ�õ������� ��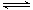 TaI4��g��+S2��g�� ����
TaI4��g��+S2��g�� ����
 TaI4(g)+S2(g) ��H��0 ����Ӧ����ƽ�ⳣ������ʽK= ����K=1����ij�����ܱ������м���1mol I2��g��������TaS2��s����I2��g����ƽ��ת����Ϊ ��
TaI4(g)+S2(g) ��H��0 ����Ӧ����ƽ�ⳣ������ʽK= ����K=1����ij�����ܱ������м���1mol I2��g��������TaS2��s����I2��g����ƽ��ת����Ϊ ��
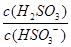 �� ���������С�����䡱����
�� ���������С�����䡱����