��Ŀ����
���ڳ�����0.1 mol��L��1��ˮ��0.1 mol��L��1���ᣬ����˵����ȷ����
| A��0.1 mol��L��1��ˮ����Һ��pH=13 |
| B��0.1 mol��L��1��ˮ��ˮϡ�ͣ���Һ��c��H+����c��OH��������С |
| C��0.1 mol��L��1������Һ�У�c��H+����c��CH3COO���� |
| D��0.1 mol��L��1������0.1 mol��L��1NaOH��Һ��������������Һ�У�c��Na+����c��CH3COO������c��OH������c��H+�� |
D
���������A����ˮ����������ʣ����ֵ���NH3
 NH4++OH�����ʳ�����0.1 mol��L��1��ˮ����Һ��pH<13��A����B����ˮ��ˮϡ�ͣ����Լ�����c��OH������С��Kw���䣬c��H+������B����C��0.1 mol��L��1������Һ�д��ڵ���غ�c��H+����c��CH3COO����+c��OH������C����D��0.1 mol��L��1������0.1 mol��L��1NaOH��Һ��������ʱ������ǡ����ȫ��Ӧ����ǿ��������CH3COONa��CH3COONa�е�CH3COO���ܷ���ˮ��ʹ����Һ���ּ��ԣ�����c��Na+����c��CH3COO������c��OH������c��H+����D��ȷ��
NH4++OH�����ʳ�����0.1 mol��L��1��ˮ����Һ��pH<13��A����B����ˮ��ˮϡ�ͣ����Լ�����c��OH������С��Kw���䣬c��H+������B����C��0.1 mol��L��1������Һ�д��ڵ���غ�c��H+����c��CH3COO����+c��OH������C����D��0.1 mol��L��1������0.1 mol��L��1NaOH��Һ��������ʱ������ǡ����ȫ��Ӧ����ǿ��������CH3COONa��CH3COONa�е�CH3COO���ܷ���ˮ��ʹ����Һ���ּ��ԣ�����c��Na+����c��CH3COO������c��OH������c��H+����D��ȷ��
��ϰ��ϵ�д�
�����Ŀ
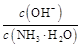 ����
����