��Ŀ����
����Ŀ�������ɶ�����Ⱦ���γ�,���������PM2.5�����������NOx����CO��SO2�ȡ���ѧ�ڽ��������Ⱦ��������Ҫ�����á�
��1����֪:��![]() ��H1=-566.0 kJ��mol-1��
��H1=-566.0 kJ��mol-1��![]() ����H2=-116.5 kJ��mol-1��
����H2=-116.5 kJ��mol-1��![]() �� ��H3=+180.5 kJ��mol-1��������NO2 ��CO ת��������Ⱦ������Ȼ�ѧ����ʽΪ____��
�� ��H3=+180.5 kJ��mol-1��������NO2 ��CO ת��������Ⱦ������Ȼ�ѧ����ʽΪ____��
��2���о���������NH3�ɳ�ȥ���Ṥҵβ���е�NO��NH3��NO�����ʵ���֮�ȷֱ�Ϊ1��2��1��1.5��3��1ʱ,NO�ѳ������¶ȱ仯��������ͼ��ʾ��
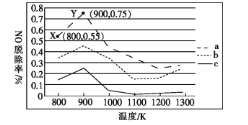
������a��,NO����ʼŨ��Ϊ6��10-4mg��m-3,��X�㵽Y�㾭��10 s,���ʱ�����NO���ѳ�����Ϊ___________mg�� m-3��s-1��
������c��Ӧ��NH3��NO�����ʵ���֮����___,��������___��
��3��̿���������е���Ҫ������,�о����������Ի������,���ɻ��,������Կ�������SO2������̵������仯ģ���������ͼ��ʾ��
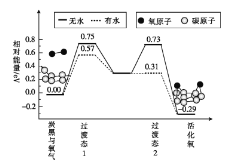
��ˮ�����,һ�������ӵĻ��Ϊ__��,��������ӵ�������___��������ˮ��������ˮ������
��4�����÷�Ӧ![]() ���ɵĵ�ؼ�����Ч��������������ŷ�,����������Ⱦ,���ܳ�����û�ѧ��,װ����ͼ��ʾ��
���ɵĵ�ؼ�����Ч��������������ŷ�,����������Ⱦ,���ܳ�����û�ѧ��,װ����ͼ��ʾ��
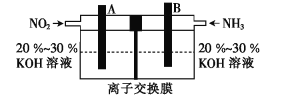
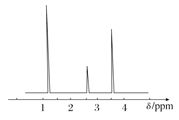
��B���ĵ缫��ӦʽΪ___��
������Ӧת��1.2 mol����,A������N2�����Ϊ___L����״������
���𰸡�2NO2��g��+4CO��g��=N2��g��+4CO2��g�� ��H=��1196.0kJ��mol-1 1.2��10��5 1��2 NH3��NO�����ʵ����ı�ֵԽ��NO�ѳ���Խ�� 0.75eV ��ˮ 2NH3-6e-+6OH-=N2+6H2O 3.36
��������
��1���ɸ�˹���ɿ�֪���١�2���ڡ��ۿɵ÷�����NO2��CO ת��������Ⱦ������Ȼ�ѧ����ʽΪ2NO2��g��+4CO��g��=N2��g��+4CO2��g�������H=��H1��2����H2����H3=��-566.0 kJ��mol-1������-116.5 kJ��mol-1������+180.5 kJ��mol-1��=��1196.0kJ��mol-1���ʴ�Ϊ��2NO2��g��+4CO��g��=N2��g��+4CO2��g�� ��H=��1196.0kJ��mol-1��
��2��������a�У�NO����ʼŨ��Ϊ6��10-4mg/m3��A����ѳ���Ϊ0.55��B����ѳ���Ϊ0.75����A�㵽B�㾭��10s����ʱ�����NO���ѳ�����=![]() =1.2��10��5 mg�� m-3��s-1���ʴ�Ϊ��1.2��10��5��
=1.2��10��5 mg�� m-3��s-1���ʴ�Ϊ��1.2��10��5��
��NH3��NO�����ʵ����ı�ֵԽ��NH3�����ʵ���Խ��NO�ѳ���Խ�����ʵ���֮�ȷֱ�Ϊ1��2��1��1.5��3��1����Ӧ�����߷ֱ�Ϊc��b��a��������c��ӦNH3��NO�����ʵ���֮����1��2���ʴ�Ϊ��1��2��NH3��NO�����ʵ����ı�ֵԽ��NO�ѳ���Խ��
��3����������ͼ������������Ӧ�Ļ��Ϊ��ܽϴ��ߣ���û��ˮ����ķ�Ӧ���ΪE=0.75eV����ˮ����ķ�Ӧ�Ļ��ΪE=0.57eV������ˮ��ʹ�����ӻ��Ӧ�Ļ�ܽ���0.75eV-0.57eV=0.18eV���ʴ�Ϊ��0.75eV����ˮ��
��4��������װ��ͼ��֪���缫Bͨ�백������KOH��Һ�а���������Ϊ�������缫��ӦʽΪ2NH3-6e-+6OH-=N2+6H2O���ʴ�Ϊ��2NH3-6e-+6OH-=N2+6H2O��
���ɻ�ѧ����ʽ��֪��A������5mol��������������3mol��������Ӧת��24mol���ӣ���Ӧת��1.2 mol���ӣ�A������0.15mol��������״��ѡ���Ϊ0.15mol��22.4L/mol=3.36L���ʴ�Ϊ��3.36��