ЬтФПФкШн
ЁОЬтФПЁПЃЈ1ЃЉNH3ОвЛЯЕСаЗДгІПЩвдЕУЕНHNO3КЭNH4NO3ЃЌШчЭМЫљЪОЃЌ

дкЂёжаЃЌNH3КЭO2дкДпЛЏМСзїгУЯТЗДгІЃЌЦфЛЏбЇЗНГЬЪНЪЧЃК__________________________ЁЁ

ЃЈ2ЃЉЂђжаЃЌ2NOЃЈgЃЉ+ O2ЃЈgЃЉ 2NO2ЃЈgЃЉЃЎдкЦфЫћЬѕМўЯрЭЌЪБЃЌЗжБ№ВтЕУNOЕФЦНКтзЊЛЏТЪдкВЛЭЌбЙЧПЃЈp1ЁЂp2ЃЉЯТЮТЖШБфЛЏЕФЧњЯпЃЈШчЭМЃЉЃЎ
Ђйp1ЁЂp2ЕФДѓаЁЙиЯЕp1 ________ p2ЃЈЬюДѓгкЁЂЕШгкЁЂаЁгкЃЉЁЁЃЎ
ЂкЫцЮТЖШЩ§ИпЃЌИУЗДгІЦНКтГЃЪ§БфЛЏЕФЧїЪЦЪЧ_________________________
ЃЈ3ЃЉЂѓжаЃЌНЕЕЭЮТЖШЃЌНЋNO2ЃЈgЃЉзЊЛЏЮЊN2O4ЃЈ1ЃЉдйжЦБИХЈЯѕЫсЃЎ
N2O4гыO2ЃЌ H2OЛЏКЯЕФЛЏбЇЗНГЬЪНЪЧ______________________________
ЃЈ4ЃЉЂєжаЃЌЕчНтNOжЦБИNH4NO3ЃЌЦфдРэдРэШчЯТЭМЫљЪОЃЌЮЊЪЙЕчНтВњЮяШЋВПзЊЛЏЮЊNH4NO3ЃЌ ашВЙГфЮяжЪ___________ ЃЌЫЕУїРэгЩЃК___________________ЃЎ
 .
.
ЁОД№АИЁП4NH3+5O2![]() 4NO+6H2O аЁгк БфаЁ 2N2O4+O2+2H2O=4HNO3 NH3 ИљОнЗДгІ8NO+7H2O
4NO+6H2O аЁгк БфаЁ 2N2O4+O2+2H2O=4HNO3 NH3 ИљОнЗДгІ8NO+7H2O![]() 3NH4NO3+2HNO3ЃЌЕчНтЩњГЩЕФHNO3ЖрЃЌЫљвдашГфШыNH3ЃЎ
3NH4NO3+2HNO3ЃЌЕчНтЩњГЩЕФHNO3ЖрЃЌЫљвдашГфШыNH3ЃЎ
ЁОНтЮіЁП
дкДпЛЏМСЁЂМгШШЬѕМўЯТЃЌАБЦјБЛбѕЛЏЩњГЩNOКЭЫЎЃЛЯрЭЌЮТЖШЯТЃЌдіДѓбЙЧПЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌдђNOЕФзЊЛЏТЪдіДѓЃЛИљОнЭМЯѓжЊЃЌЯрЭЌбЙЧПЯТЃЌЩ§ИпЮТЖШЃЌNOзЊЛЏТЪМѕаЁЃЌЫЕУїе§ЗДгІЪЧЗХШШЗДгІЃЌОнДЫХаЖЯЛЏбЇЦНКтГЃЪ§гыЮТЖШЕФЙиЯЕЃЛN2O4ЁЂO2КЭH2OЗДгІЩњГЩЯѕЫсЃЛЕчНтNOжЦБИNH4NO3ЃЌбєМЋЗДгІЮЊNO-3e-+2H2O=NO3-+4H+ЃЌвѕМЋЗДгІЮЊЃКNO+5e-+6H+=NH4++H2OЃЌИљОнзЊвЦЕчзгЪиКуХаЖЯашвЊМгШыЮяжЪЁЃ
(1)АБЕФДпЛЏбѕЛЏЕФЗДгІЗНГЬЪНЮЊ4NH3ЃЋ5O2![]() 4NOЃЋ6H2OЁЃ(2)ЂйгЩ2NO(g)ЃЋO2(g)
4NOЃЋ6H2OЁЃ(2)ЂйгЩ2NO(g)ЃЋO2(g)![]() 2NO2(g)ПЩжЊИУЗДгІЮЊЦјЬхЬхЛ§МѕаЁЕФЗДгІЃЌЮТЖШЯрЭЌЃЌдіДѓбЙЧПЃЌЦНКте§ЯђвЦЖЏЃЌNOЕФЦНКтзЊЛЏТЪдіДѓЃЌИљОнЭМЪОКЭзјБъКЌвхЃЌХаЖЯp1<p2ЃЛЂкдйПДЭЌвЛбЙЧПЯпЃЌЮТЖШЩ§ИпЃЌNOЕФЦНКтзЊЛЏТЪНЕЕЭЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌдђе§ЗДгІЮЊЗХШШЗДгІЃЌЮТЖШЩ§ИпЃЌЦНКтГЃЪ§МѕаЁЁЃЃЈ3ЃЉN2O4ЁЂO2КЭH2OЗДгІЩњГЩЯѕЫсЕФЗДгІЗНГЬЪНЮЊ2N2O4ЃЋ O2ЃЋ2H2O=4HNO3ЁЃ(4)ИљОнЙЄзїдРэзАжУЭМЃЌПЩвдШЗЖЈбєМЋЮЊNOЪЇШЅЕчзгзЊБфЮЊNO3ЁЊЃЌвѕМЋNOзЊБфЮЊNH4+ЃЌИљОнЕчМЋЗДгІЪщаДЕчМЋЗДгІЪНЮЊЃКбєМЋЃКNOЃ3eЃЃЋ2H2O= NO3ЁЊЃЋ4HЃЋЃЌвѕМЋЃКNOЃЋ5eЃЃЋ 6HЃЋ= NH4+ЃЋ H2OЃЌШЛКѓИљОнЕУЪЇЕчзгЪиКуЃЌЯѕЫсИљРызгЮяжЪЕФСПБШяЇИљРызгЮяжЪЕФСПЖрЃЌЫљвдашвЊЯђШмвКжаМгШыЕФЮяжЪЮЊNH3(МД8NOЃЋ7H2O=3NH4NO3ЃЋ2HNO3)ЁЃ
2NO2(g)ПЩжЊИУЗДгІЮЊЦјЬхЬхЛ§МѕаЁЕФЗДгІЃЌЮТЖШЯрЭЌЃЌдіДѓбЙЧПЃЌЦНКте§ЯђвЦЖЏЃЌNOЕФЦНКтзЊЛЏТЪдіДѓЃЌИљОнЭМЪОКЭзјБъКЌвхЃЌХаЖЯp1<p2ЃЛЂкдйПДЭЌвЛбЙЧПЯпЃЌЮТЖШЩ§ИпЃЌNOЕФЦНКтзЊЛЏТЪНЕЕЭЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌдђе§ЗДгІЮЊЗХШШЗДгІЃЌЮТЖШЩ§ИпЃЌЦНКтГЃЪ§МѕаЁЁЃЃЈ3ЃЉN2O4ЁЂO2КЭH2OЗДгІЩњГЩЯѕЫсЕФЗДгІЗНГЬЪНЮЊ2N2O4ЃЋ O2ЃЋ2H2O=4HNO3ЁЃ(4)ИљОнЙЄзїдРэзАжУЭМЃЌПЩвдШЗЖЈбєМЋЮЊNOЪЇШЅЕчзгзЊБфЮЊNO3ЁЊЃЌвѕМЋNOзЊБфЮЊNH4+ЃЌИљОнЕчМЋЗДгІЪщаДЕчМЋЗДгІЪНЮЊЃКбєМЋЃКNOЃ3eЃЃЋ2H2O= NO3ЁЊЃЋ4HЃЋЃЌвѕМЋЃКNOЃЋ5eЃЃЋ 6HЃЋ= NH4+ЃЋ H2OЃЌШЛКѓИљОнЕУЪЇЕчзгЪиКуЃЌЯѕЫсИљРызгЮяжЪЕФСПБШяЇИљРызгЮяжЪЕФСПЖрЃЌЫљвдашвЊЯђШмвКжаМгШыЕФЮяжЪЮЊNH3(МД8NOЃЋ7H2O=3NH4NO3ЃЋ2HNO3)ЁЃ

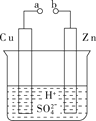
ЁОЬтФПЁПдЕчГиЪЧЛЏбЇЖдШЫРрЕФвЛЯюжиДѓЙБЯзЁЃ
(1)ФГаЫШЄаЁзщЮЊбаОПЕчГидРэЃЌЩшМЦШчЭМAзАжУЁЃ
|
|
A | B |
ЂйaКЭbВЛСЌНгЪБЃЌЩеБжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЪЧ___________________ЁЃ
ЂкaКЭbгУЕМЯпСЌНгЃЌCuМЋЮЊдЕчГи________(ЬюЁАе§ЁБЛђЁАИКЁБ)МЋЃЌИУЕчМЋЗДгІЪНЪЧ____________________________ЁЃ
ЂлЮоТлaКЭbЪЧЗёСЌНгЃЌZnЦЌОљБЛИЏЪДЃЌШєзЊвЦСЫ0.4 molЕчзгЃЌдђРэТлЩЯZnЦЌжЪСПМѕЧс________ gЁЃ
(2)ШчЭМBЪЧМзЭщШМСЯЕчГидРэЪОвтЭМЃЌЛиД№ЯТСаЮЪЬтЃК
ЂйЕчГиЕФИКМЋЪЧ________(ЬюЁАaЁБЛђЁАbЁБ)ЕчМЋЃЌИУМЋЕФЕчМЋЗДгІЪНЮЊ________________ЁЃ
ЂкЕчГиЙЄзївЛЖЮЪБМфКѓЕчНтжЪШмвКЕФpH________(ЬюЁАдіДѓЁБЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЁЃ
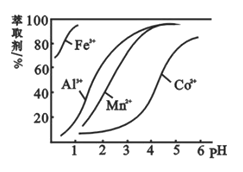
ЁОЬтФПЁПВнЫсюмПЩгУгкжИЪОМСКЭДпЛЏМСЕФжЦБИЁЃгУЫЎюмПѓ(жївЊГЩЗжЮЊCo2O3ЃЌКЌЩйСПFe2O3ЁЂAl2O3ЁЂMnOЁЂMgOЁЂCaOЁЂSiO2ЕШ)жЦШЁCoC2O4ЁЄ2H2OЙЄвеСїГЬШчЯТЃК
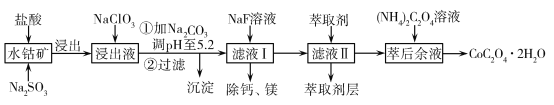
вбжЊЃКЂйНўГівККЌгаЕФбєРызгжївЊгаHЃЋЁЂCo2ЃЋЁЂFe2ЃЋЁЂMn2ЃЋЁЂCa2ЃЋЁЂMg2ЃЋЁЂAl3ЃЋЕШЃЛ
ЂкЫсадЬѕМўЯТЃЌClOВЛЛсбѕЛЏCo2ЃЋЃЌClOзЊЛЏЮЊClЃЃЛЂлВПЗжбєРызгвдЧтбѕЛЏЮяаЮЪНГСЕэЪБШмвКЕФpHМћБэЃК
ГСЕэЮя | Fe(OH)3 | Al(OH)3 | Co(OH)2 | Fe(OH)2 | Mn(OH)2 |
ЭъШЋГСЕэЕФpH | 3.7 | 5.2 | 9.2 | 9.6 | 9.8 |
ЃЈ1ЃЉНўГіЙ§ГЬжаМгШыNa2SO3ЕФжївЊФПЕФЪЧ________ЁЃ
ЃЈ2ЃЉЯђНўГівКжаМгШыNaClO3ЕФРызгЗДгІЗНГЬЪНЃК________ЁЃ
ЃЈ3ЃЉвбжЊЃКГЃЮТЯТNH3ЁЄH2O![]() NH4+ЃЋOHЃЁЁKbЃН1.8ЁС10Ѓ5
NH4+ЃЋOHЃЁЁKbЃН1.8ЁС10Ѓ5
H2C2O4![]() HЃЋЃЋHC2O4-ЁЁKa1ЃН5.4ЁС10Ѓ2ЁЁHC2O4-
HЃЋЃЋHC2O4-ЁЁKa1ЃН5.4ЁС10Ѓ2ЁЁHC2O4-![]() HЃЋЃЋC2O42-ЁЁKa2ЃН5.4ЁС10Ѓ5
HЃЋЃЋC2O42-ЁЁKa2ЃН5.4ЁС10Ѓ5
дђИУСїГЬжаЫљгУ(NH4)2C2O4ШмвКЕФpH________7(ЬюЁАЃОЁБЛђЁАЃМЁБЛђЁАЃНЁБ)ЁЃ
ЃЈ4ЃЉМгШы(NH4)2C2O4 ШмвККѓЮіГіОЇЬхЃЌдйЙ§ТЫЁЂЯДЕгЃЌЯДЕгЪБПЩбЁгУЕФЪдМСгаЃК________ЁЃ
AЃЎеєСѓЫЎ BЃЎздРДЫЎ
CЃЎБЅКЭЕФ(NH4)2C2O4ШмвК DЃЎЯЁбЮЫс
ЃЈ5ЃЉнЭШЁМСЖдН№ЪєРызгЕФнЭШЁТЪгыpHЕФЙиЯЕШчЭМЃЌнЭШЁМСЕФзїгУЪЧ________ЃЛЦфЪЙгУЕФЪЪвЫpHЗЖЮЇЪЧ________ЁЃ
AЃЎ2.0ЁЋ2.5 BЃЎ3.0ЁЋ3.5 CЃЎ4.0ЁЋ4.5