��Ŀ����
 �������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�ʹ������������������ⶼ������A�����֣�
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�ʹ������������������ⶼ������A�����֣�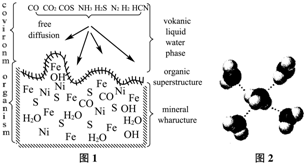
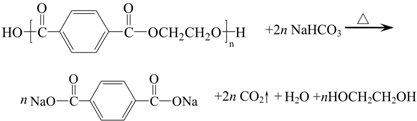
A��������Һ�о���ͼ1�����ڵ�����е�ǰ�أ�������Һ������̴ѡ�����Χ������FeS����ͭ��п��ȿ��
��1��Ni2+�ĺ�������Ų�ʽ��
��2�������±���ͭ�ĵ�һ�����ܣ�I1��С��п�ĵ�һ�����ܣ���ͭ�ĵڶ������ܣ�I2��ȴ����п�ĵڶ������ܣ�����Ҫԭ����
| ������/kJ?mol-1 | I1 | I2 |
| ͭ | 746 | 1958 |
| п | 906 | 1733 |
A���縺�ԣ�N��O��S��C����������������B��CO2��COS�����ʣ���Ϊ�ȵ�����
C��NH3�����е�ԭ�Ӳ���sp3�ӻ� D��CO��H2S��HCN���Ǽ��Է���
��4����������Һ���д�������һ�������ӣ��ṹ��ͼ2�����Ļ�ѧʽΪ
��5��FeS��NaCl��Ϊ���Ӿ��壬�������ƣ�ǰ���۵�Ϊ985�棬����801�棬��ԭ����
B���Ʊ�KNO3�����ʵ�������ýᾧ���ؽᾧ����KNO3��NaCl�Ļ������з��룮������ij��ѧ��ȤС��Ļ��¼��
| NaNO3 | KNO3 | NaCl | KCl | |
| 10�� | 80.5 | 20.9 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |
ʵ�鷽����
���ܽ⣺��ȡ29.8g KCl��34.0g NaNO3����250mL�ձ��У��ټ���70.0g����ˮ�����Ȳ����裬ʹ����ȫ���ܽ⣮
�������ᾧ���������Ⱥͽ��裬����Һ����Ũ������100��ʱ������50.0g ˮ��ά�ָ��¶ȣ��ڱ���©������ͼ3��ʾ���г��ȹ��������ľ��壮�þ���m1g��
����ȴ�ᾧ������Һ��ȴ�����£�ʵ��ʱ����Ϊ10�棩���м�ѹ���ˣ���KNO3�ֲ�Ʒm2g��
�����ؽᾧ�����ֲ�Ʒȫ������ˮ���Ƴ�100��ı�����Һ����ȴ�����º���ˣ���KNO3��Ʒ��
�ٶ����������ʱ��Ӱ����Ե��ܽ�ȣ��ڸ��ֹ��˲��������У��ܼ�����ĺ��Բ��ƣ��Իش��й����⣺
��1���������г��ȹ��˵�Ŀ���ǣ�
��2�����������гн���Һ���ձ��в���������ˮ�����������ڲ������пɵôֲ�Ʒ������m2=
��3���������в��ü�ѹ���ˣ����ŵ���
������A��1��������28��Ԫ�أ���������������ԭ��ʧȥ2�����ӵõ��ģ����ݹ���ԭ������д�������ӵĺ�������Ų�ʽ��ע��ԭ��ʧȥ�����Ǵ�������ʧ��
��2��������ṹ�Ƿ��ȶ��жϣ�
��3��A������Ԫ���������ж�Ԫ�صĵ縺�ԣ�ͬһ���ڣ�ԭ������Խ�縺��Խ��ͬһ���壬ԭ������Խ�縺��ԽС��
B���ȵ����庬����ͬ��ԭ�Ӻͼ۵�������
C�����ݷ��ӵĽṹ�ж��ӻ���ʽ��
D�����ݹ��ۻ�����ṹ�жϷ��ӵ����ͣ�
��4������ͼƬ�������д���仯ѧʽ��
��5�����Ӿ����о�����Խ�����۵�Խ�ߣ������������йأ����Խ����Խ�������Ȼ��Ƶľ����жϣ�
B��1�����ݲ�ͬ�¶�����ص��ܽ�Ȳ�ͬ�������¶Ƚϵ�ʱ����������ܽ�ȱ�С��������
��2���ȸ����Ȼ��غ������Ƶ��������������ʵ��������Ȼ��غ������ƵĻ����Һ�����Ȼ��ƺ�����صĻ����Һ������100��ʱ���Ȼ��ƺ�����ص��ܽ���ж��Ƿ������Ȼ��ƺ�����ؼ������Ĺ����������ٸ���10��ʱ�Ȼ��ƺ�����ص��ܽ���ж��Ƿ��й��������������Ĺ���������
��3����ѹ�IJ����ŵ��ǣ��ɼӿ�����ٶȣ����ܵõ��ϸ���ij���������ͼƬ�ж�������������ʵ������з��ֵ�������Ӧ��ȡ�Ĵ�ʩ�Ǵ�����K��
��2��������ṹ�Ƿ��ȶ��жϣ�
��3��A������Ԫ���������ж�Ԫ�صĵ縺�ԣ�ͬһ���ڣ�ԭ������Խ�縺��Խ��ͬһ���壬ԭ������Խ�縺��ԽС��
B���ȵ����庬����ͬ��ԭ�Ӻͼ۵�������
C�����ݷ��ӵĽṹ�ж��ӻ���ʽ��
D�����ݹ��ۻ�����ṹ�жϷ��ӵ����ͣ�
��4������ͼƬ�������д���仯ѧʽ��
��5�����Ӿ����о�����Խ�����۵�Խ�ߣ������������йأ����Խ����Խ�������Ȼ��Ƶľ����жϣ�
B��1�����ݲ�ͬ�¶�����ص��ܽ�Ȳ�ͬ�������¶Ƚϵ�ʱ����������ܽ�ȱ�С��������
��2���ȸ����Ȼ��غ������Ƶ��������������ʵ��������Ȼ��غ������ƵĻ����Һ�����Ȼ��ƺ�����صĻ����Һ������100��ʱ���Ȼ��ƺ�����ص��ܽ���ж��Ƿ������Ȼ��ƺ�����ؼ������Ĺ����������ٸ���10��ʱ�Ȼ��ƺ�����ص��ܽ���ж��Ƿ��й��������������Ĺ���������
��3����ѹ�IJ����ŵ��ǣ��ɼӿ�����ٶȣ����ܵõ��ϸ���ij���������ͼƬ�ж�������������ʵ������з��ֵ�������Ӧ��ȡ�Ĵ�ʩ�Ǵ�����K��
����⣺��1��������28��Ԫ�أ���ԭ�Ӻ�����28�����ӣ���������������ԭ��ʧȥ2�����ӵõ��ģ����ݹ���ԭ������д�������ӵĺ�������Ų�ʽ��Ni2+�ĺ�������Ų�ʽ��1s22s22p63s23p63d 8��
�ʴ�Ϊ��1s22s22p63s23p63d8��[Ar]3d8��
��2��Cuʧȥһ�����ӳ�ΪCu+����������Ų���[Ar]3d104s1��Ϊ[Ar]3d10�����ϵ͵��ȶ��ṹ����Zn��������Ų�Ϊ[Ar]3d104s2���ȶ�������Cu��һ�����ܽ�ZnС���ڶ���������Խϴ�
�ʴ�Ϊ��Cuʧȥһ�����ӳ�ΪCu+����������Ų���[Ar]3d104s1��Ϊ[Ar]3d10�����ϵ͵��ȶ��ṹ����Zn��������Ų�Ϊ[Ar]3d104s2���ȶ�������Cu��һ�����ܽ�ZnС���ڶ���������Խϴ�
��3��A��ͬһ���ڣ�ԭ������Խ�縺��Խ��ͬһ���壬ԭ������Խ�縺��ԽС�����Ե縺�Դ�СΪ
O��N��S��C����A����
B��CO2��COS�����ʣ���ԭ�Ӹ�����ȣ�������ļ۵�������ȶ���6�����Ի�Ϊ�ȵ����壬��B��ȷ��
C��NH3�����е�ԭ�Ӳ���sp3�ӻ��������������Σ���C��ȷ��
D��CO��H2S��HCN�����ǶԳ��ͽṹ�����Զ��Ǽ��Է��ӣ���D��ȷ��
��ѡ��BCD��
��4������ͼƬ֪���������к���11����ԭ�ӣ�5����ԭ�ӣ����Ǵ�һ����λ����ɵ����ӣ����Ը�����ΪH11O5+��
�ʴ�Ϊ��H11O5+��
��5��FeS�����ĵ�ɱ��Ȼ��ƵĶ࣬����FeS����ľ����ܱ�NaCl���徧���ܴ�����FeS������۵��NaCl����Ĵ����Ȼ��Ƶ��ж�֪��Ϊ�������壮
�ʴ�Ϊ��FeS����ľ����ܱ�NaCl���徧���ܴ��������壮
B����1���¶Ƚϵ�ʱ����������ܽ�ȱ�С��������Ϊ��ֹ����ʱ��������ض����Ͳ��ʣ�����Ҫ���ȹ��ˣ�
�ʴ�Ϊ����ֹ����ʱ��������ض����Ͳ��ʣ�
��2����29.8g KCl�����ʵ���=
=0.4mol��34.0g NaNO3�����ʵ���=
=0.4mol
�Ȼ��غ������Ʋ���Ӧ�������Ȼ��غ������ƵĻ����Һ�ɿ������Ȼ��ƺ�����صĻ����Һ������غ��Ȼ��Ƶ����ʵ�������0.4mol������ص�����Ϊ40.4g���Ȼ��Ƶ�����Ϊ23.4g��
100��ʱ��20gˮ�������Ȼ��Ƶ�����Ϊ39.1g��
=7.82g��23.4g�����������Ȼ��Ƶ�����=23.4g-7.82g=15.58g��
20gˮ����������ص�����Ϊ246g��
=49.2g��40.4g������������γɵ��Dz�������Һ����Һ�����Ĺ���ȫ�����Ȼ��ƣ���m1g=15.58g��
��10��ʱ��20gˮ�������Ȼ��Ƶ�����Ϊ35.7g��
=7.14g�����������Ȼ��Ƶ�����Ϊ7.82g-7.14g=0.68g��
20gˮ����������ص�����Ϊ20.9g��
=4.18g��40.4g��������������ص�����Ϊ40.4g-4.18g=36.22g��
�������������������Ϊ0.68g+36.22g=36.9g��
10��ʱ����0.68g�Ȼ�������ˮ�õ�������Һ����Ҫˮ������Ϊx��
=
x=1.9g��
�ʴ�Ϊ��36.9��0.68��1.90��
��3����ѹ�IJ����ŵ��ǣ��ɼӿ�����ٶȣ����ܵõ��ϸ���ij�����
����ͼƬ֪�����ò�������Ϊ����ƿ����ȫƿ�������ã���ʵ������з��ֵ�������Ӧ��ȡ�Ĵ�ʩ�Ǵ�����K��
�ʴ�Ϊ���ӿ�����ٶȣ����ܵõ��ϸ���ij���������ƿ����ȫƿ�������ã�������K��
�ʴ�Ϊ��1s22s22p63s23p63d8��[Ar]3d8��
��2��Cuʧȥһ�����ӳ�ΪCu+����������Ų���[Ar]3d104s1��Ϊ[Ar]3d10�����ϵ͵��ȶ��ṹ����Zn��������Ų�Ϊ[Ar]3d104s2���ȶ�������Cu��һ�����ܽ�ZnС���ڶ���������Խϴ�
�ʴ�Ϊ��Cuʧȥһ�����ӳ�ΪCu+����������Ų���[Ar]3d104s1��Ϊ[Ar]3d10�����ϵ͵��ȶ��ṹ����Zn��������Ų�Ϊ[Ar]3d104s2���ȶ�������Cu��һ�����ܽ�ZnС���ڶ���������Խϴ�
��3��A��ͬһ���ڣ�ԭ������Խ�縺��Խ��ͬһ���壬ԭ������Խ�縺��ԽС�����Ե縺�Դ�СΪ
O��N��S��C����A����
B��CO2��COS�����ʣ���ԭ�Ӹ�����ȣ�������ļ۵�������ȶ���6�����Ի�Ϊ�ȵ����壬��B��ȷ��
C��NH3�����е�ԭ�Ӳ���sp3�ӻ��������������Σ���C��ȷ��
D��CO��H2S��HCN�����ǶԳ��ͽṹ�����Զ��Ǽ��Է��ӣ���D��ȷ��
��ѡ��BCD��
��4������ͼƬ֪���������к���11����ԭ�ӣ�5����ԭ�ӣ����Ǵ�һ����λ����ɵ����ӣ����Ը�����ΪH11O5+��
�ʴ�Ϊ��H11O5+��
��5��FeS�����ĵ�ɱ��Ȼ��ƵĶ࣬����FeS����ľ����ܱ�NaCl���徧���ܴ�����FeS������۵��NaCl����Ĵ����Ȼ��Ƶ��ж�֪��Ϊ�������壮
�ʴ�Ϊ��FeS����ľ����ܱ�NaCl���徧���ܴ��������壮
B����1���¶Ƚϵ�ʱ����������ܽ�ȱ�С��������Ϊ��ֹ����ʱ��������ض����Ͳ��ʣ�����Ҫ���ȹ��ˣ�
�ʴ�Ϊ����ֹ����ʱ��������ض����Ͳ��ʣ�
��2����29.8g KCl�����ʵ���=
| 29.8g |
| 74.5g/mol |
| 34.0g |
| 85g/mol |
�Ȼ��غ������Ʋ���Ӧ�������Ȼ��غ������ƵĻ����Һ�ɿ������Ȼ��ƺ�����صĻ����Һ������غ��Ȼ��Ƶ����ʵ�������0.4mol������ص�����Ϊ40.4g���Ȼ��Ƶ�����Ϊ23.4g��
100��ʱ��20gˮ�������Ȼ��Ƶ�����Ϊ39.1g��
| 1 |
| 5 |
20gˮ����������ص�����Ϊ246g��
| 1 |
| 5 |
��10��ʱ��20gˮ�������Ȼ��Ƶ�����Ϊ35.7g��
| 1 |
| 5 |
20gˮ����������ص�����Ϊ20.9g��
| 1 |
| 5 |
�������������������Ϊ0.68g+36.22g=36.9g��
10��ʱ����0.68g�Ȼ�������ˮ�õ�������Һ����Ҫˮ������Ϊx��
| 0.68 |
| x |
| 35.7 |
| 100 |
x=1.9g��
�ʴ�Ϊ��36.9��0.68��1.90��
��3����ѹ�IJ����ŵ��ǣ��ɼӿ�����ٶȣ����ܵõ��ϸ���ij�����
����ͼƬ֪�����ò�������Ϊ����ƿ����ȫƿ�������ã���ʵ������з��ֵ�������Ӧ��ȡ�Ĵ�ʩ�Ǵ�����K��
�ʴ�Ϊ���ӿ�����ٶȣ����ܵõ��ϸ���ij���������ƿ����ȫƿ�������ã�������K��
���������⿼����ԭ�Ӻ�������Ų����縺�ԡ������ܡ����ʵķ�����ᴿ��֪ʶ�㣬�ۺ��Խ�ǿ���ѶȽϴ�����ʱע������Ų�ʽ����д������ע����ԭ��ʧȥ������������ʱ������ӵ�ʧȥ�Ⱥ�˳��Ϊ����������ʧȥ���ӣ����Ǹ�������ʧȥ���ӣ�

��ϰ��ϵ�д�
�����Ŀ
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֣�
A����������Raney-Ni����һ����ʷ�ƾá�Ӧ�ù㷺�Ĵ���������-���Ͻ�Ϊԭ���Ƶã�
��1��Ԫ�ص�һ�����ܣ�Al Mg��ѡ�������������������=����
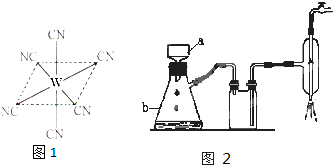
��2������������һʵ��Ϊ�� ������b�н���sp
������b�н���sp 3�ӻ���ԭ���У� ��
3�ӻ���ԭ���У� ��
��3��һ�������Ͻ�Ľṹ����ͼ������ṹ���ƵĻ������ǣ� ��ѡ����ţ�a���Ȼ��� b���Ȼ�� c��ʯӢ d�����ʯ����
��4��ʵ���Ҽ���Ni2+���ö���ͪ���֮���������Ⱥ�ɫ����������
��Ni2+�ڻ�̬ʱ����������Ų�ʽΪ�� ��
������������û�ѧ����������δ��������������������λ��Ϊ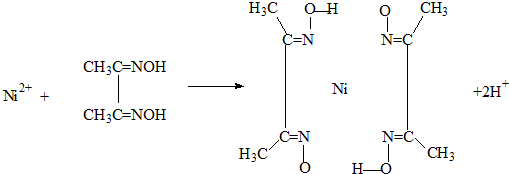
B��4-�ȱ����Ǻϳ�����ѧ�Լ���Ⱦ�ϡ�ɫ�صȻ�����Ʒ���Ʊ�4-�ȱ�����ԭ�����£�
 ����1L�ܱ������м���4-��������100g�����������������������������������Ƽ���
����1L�ܱ������м���4-��������100g�����������������������������������Ƽ��� �����״�250mL���ܼ������ܷ⣻�ȳ��뵪����Ȼ�����������һ��ѹ��������������ѹ�����¶ȵ������¼��ⷴӦ����ַ�Ӧ����ȴ�����ˣ�ϴ�ӣ������ռ�64��65����֣�
�����״�250mL���ܼ������ܷ⣻�ȳ��뵪����Ȼ�����������һ��ѹ��������������ѹ�����¶ȵ������¼��ⷴӦ����ַ�Ӧ����ȴ�����ˣ�ϴ�ӣ������ռ�64��65����֣�
��1����ʼʱ��ͨ��N2��Ŀ���� ��
��2������ʵ���й��˵�Ŀ���� ��ϴ�����õ��Լ��� ��
��3������ʱ���õ������ܡ���ƿ���¶ȼ��⣬���õ��IJ��������У� �� �����Һ����Ҫ�ɷ��� ��
��4���������¶ȡ�����ѹ�������������������������Է�Ӧ��Ӱ�죮
�Ż��������ǣ��¶ȡ�����ѹ������������������������ѡ������������ ��
A����������Raney-Ni����һ����ʷ�ƾá�Ӧ�ù㷺�Ĵ���������-���Ͻ�Ϊԭ���Ƶã�
��1��Ԫ�ص�һ�����ܣ�Al
��2������������һʵ��Ϊ��
 ������b�н���sp
������b�н���sp 3�ӻ���ԭ���У�
3�ӻ���ԭ���У���3��һ�������Ͻ�Ľṹ����ͼ������ṹ���ƵĻ������ǣ�
��4��ʵ���Ҽ���Ni2+���ö���ͪ���֮���������Ⱥ�ɫ����������
��Ni2+�ڻ�̬ʱ����������Ų�ʽΪ��
������������û�ѧ����������δ��������������������λ��Ϊ

B��4-�ȱ����Ǻϳ�����ѧ�Լ���Ⱦ�ϡ�ɫ�صȻ�����Ʒ���Ʊ�4-�ȱ�����ԭ�����£�
 ����1L�ܱ������м���4-��������100g�����������������������������������Ƽ���
����1L�ܱ������м���4-��������100g�����������������������������������Ƽ��� �����״�250mL���ܼ������ܷ⣻�ȳ��뵪����Ȼ�����������һ��ѹ��������������ѹ�����¶ȵ������¼��ⷴӦ����ַ�Ӧ����ȴ�����ˣ�ϴ�ӣ������ռ�64��65����֣�
�����״�250mL���ܼ������ܷ⣻�ȳ��뵪����Ȼ�����������һ��ѹ��������������ѹ�����¶ȵ������¼��ⷴӦ����ַ�Ӧ����ȴ�����ˣ�ϴ�ӣ������ռ�64��65����֣���1����ʼʱ��ͨ��N2��Ŀ����
��2������ʵ���й��˵�Ŀ����
��3������ʱ���õ������ܡ���ƿ���¶ȼ��⣬���õ��IJ��������У�
��4���������¶ȡ�����ѹ�������������������������Է�Ӧ��Ӱ�죮
| ��1�¶� | ��2����ѹ�� | ��3�������� | ��4�������� | |||||||||||||||
| ��� | �¶�/�� | ת����/% | ѡ�� ��/% |
��Ӧʱ��/h | ��� | ����ѹ��/MPa | ѡ����/% | ��Ӧʱ��/h | ��� | ����������/g | ѡ����/% | ��Ӧʱ��/h | ��� | ��������/% | ѡ����/% | |||
| �� | 40 | δ��ȫ | 99.6 | 6 | �� | 0.5 | 99.6 | 3.7 | �� | 2 | 98.25 | 5 | �� | 0.0 | 84.3 | |||
| �� | 60 | 100 | 99.7 | 4 | �� | 1.0 | 99.7 | 2 | �� | 4 | 99.20 | 2.2 | �� | 0.3 | 99.3 | |||
| �� | 80 | 100 | 99.6 | 2.45 | �� | 1.5 | 99.2 | 1.6 | �� | 6 | 99.60 | 1.9 | �� | 0.5 | 99.7 | |||
| �� | 100 | 100 | 99.6 | 2 | �� | 2.0 | 96.4 | 1.15 | �� | 8 | 99.60 | 1.4 | �� | 0.7 | 99.6 | |||
| �� | 120 | 100 | 98.6 | 1.7 | �� | �� | 10 | 99.10 | 1.4 | �� | 1.2 | 99.7 | ||||||
 �������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֣�
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֣�