��Ŀ����
����Ŀ����1��______molH2O�к��е���ԭ������1.5molCO2�к��е���ԭ������ȡ�
��2���������ʵ�����NH3��CH4��ϣ����������NH3��CH4��������Ϊ________��
��3��ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ__________��
��4����״���£��ܶ�Ϊ0.75g��L��1��NH3��CH4��ɵĻ�������У�NH3���������Ϊ__________���û�����������������ܶ�Ϊ________��
��5����֪agA��bgBǡ����ȫ��Ӧ����0.2molC��dgD����C��Ħ������Ϊ__________��
���𰸡� 3 17��16 4��3 80% 8.4 5(a+b-d)g/mol
��������(1)������ԭ����Ŀ��ȣ���ˮ�����ʵ���Ϊ![]() =3mol���ʴ�Ϊ��3��
=3mol���ʴ�Ϊ��3��
(2)����m=nM��֪�������ʵ�����NH3��CH4��������Ϊ17g/mol��16g/mol=17��16���ʴ�Ϊ��17��16��
(3)ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���![]() =
=![]() �����
�����![]() =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ![]() ��
��
(4)�������ƽ��Ħ������Ϊ0.75gL-1��22.4L/mol=16.8g/mol����NH3���������Ϊx����17x+16(1-x)=16.8�����x=0.8=80%���û�����������������ܶ�Ϊ![]() =8.4���ʴ�Ϊ��80%��8.4��
=8.4���ʴ�Ϊ��80%��8.4��
(5)���������غ㶨�ɣ�C������Ϊ(a+b-d)g����C��Ħ������Ϊ![]() =5(a+b-d)g/mol���ʴ�Ϊ��5(a+b-d)g/mol��
=5(a+b-d)g/mol���ʴ�Ϊ��5(a+b-d)g/mol��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�����Ŀ��ijͬѧ����ͼ����ʵ�飬��A�зŵ��Ǹ���ĺ�ɫֽ����B�зŵ���ʪ��ĺ�ɫֽ����C��ʢ�ŵ�������������Һ����ش��������⡣
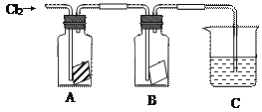
��1��ͨ��Cl2һ��ʱ���A��Bװ���У���ɫֽ��������ͽ���Ϊ��
װ�� | ���� | ���� |
A | ______________ | ��ʪ������______�����ܻ��ܣ�ʹ��ɫֽ����ɫ����Ϊ��Ӧ���ɵ�____________����Ư���� |
B | ______________ |
��2��Cװ�õ�������__________________________
д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��_________________________________