��Ŀ����
����������ָ����Һ��һ���ܴ����������( )
�ټ���Al�ܷų�H2����Һ�У�Fe2+��Al3+��NO3-��Cl-��S2-
����PH=11����Һ�У�Na+����Al(OH)4��-��NO3-��S2-��SO32-
����ˮ�����c(H+)=10-12mol��L-1����Һ�У�Cl-��HCO3-��NO3-��NH4+��F-
�ܼ���Mg�ܷų�H2����Һ�У�Mg2+��NH4+��Cl-��K+��SO42-
�ټ���Al�ܷų�H2����Һ�У�Fe2+��Al3+��NO3-��Cl-��S2-
����PH=11����Һ�У�Na+����Al(OH)4��-��NO3-��S2-��SO32-
����ˮ�����c(H+)=10-12mol��L-1����Һ�У�Cl-��HCO3-��NO3-��NH4+��F-
�ܼ���Mg�ܷų�H2����Һ�У�Mg2+��NH4+��Cl-��K+��SO42-
| A���ڢ� | B���٢ڢ� | C���٢ۢ� | D���٢� |
A
������������м���Al�ܷų�H2����Һ�������Ի���ԣ�����Fe2+��Al3+��S2-һ�����ܴ������ڡ�������ˮ�����c(H+)=10-12mol��L-1����Һ�������Ի���ԣ�����HCO3-��NH4+��F-һ�����ܴ������ڡ���ѡA��
���������Ӳ��ܴ��������һ������ǣ���1���ܷ������ֽⷴӦ������֮�䣨�����ɳ��������壬ˮ�����ᡢ������ѵ������ʣ�����2�������������������֮�䣨�磺Ca2+�� SO42-��Ag+�� SO42-������3������ȫˮ�������֮�䣬���Ԫ����������ε����������ӣ��磺Al3+�� Fe3+�� CO32-��HCO3-��AlO2-��ClO-��S2-�ȣ�����4���ܷ���������ԭ��Ӧ������֮�䣨�磺Fe ��H+��NO3-��S2-��ClO-��S2-�� Fe3+�ȣ�����5���ܷ�����Ϸ�Ӧ������֮�䣨�� Fe3+�� SCN-����������ӹ�������ʱ��Ӧ��ע����Ŀ����������������Ŀ������������һ���У�1����Һ������ԣ��ݴ����ж���Һ���Ƿ��д����� H+��OH-����2����Һ����ɫ������ɫʱ���ų� Cu2+�� Fe2+��Fe3+��MnO4-����ɫ���ӵĴ��ڣ���3����Һ�ľ��巴Ӧ�������硰������ԭ��Ӧ�������������۲�������������4���ǡ����ܡ����棬���ǡ�һ��������ȡ�

��ϰ��ϵ�д�
�����Ŀ
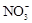
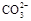
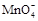 K+
K+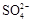 Na+
Na+ Na+
Na+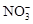 Cl�C
Cl�C