��Ŀ����
��9�֣�A��B��C��X��Ϊ��ѧ�����Ĵ��������֮��������ת����ϵ������������ȥ����
��1����X�ǿ����д��ڵ�ǿ��������ɫ���嵥�ʣ���A������___________������ţ�
a��C b��Na c��S d��Al
��2����X���ճ���������õĽ������ʣ�A�����嵥�ʣ���C��ˮ��Һ�еμ�AgNO3��Һ������������ϡHNO3�İ�ɫ������X������A��ȼ�ղ����ػ�ɫ���̡�
B�Ļ�ѧʽΪ____________�� ��Ӧ�ڵĻ�ѧ����ʽΪ�� ,
C��Һ�м���NaOH��Һ������������� ������ʽΪ ��
��9�֣�
��1��a b c
��2��FeCl3; 2 FeCl3 + Fe = 3 FeCl2 ;�����ɰ�ɫ�������ܿ��ɻ��̣����ձ�ɺ��ɫ��
4Fe(OH)2 + O2 + 2 H2O = 4 Fe(OH)3
����:��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ



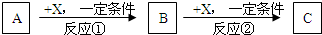

 ����3�ֻ�����A��B��C����������Ԫ��R����ת����ϵ��ͼ��ʾ��
����3�ֻ�����A��B��C����������Ԫ��R����ת����ϵ��ͼ��ʾ��