��Ŀ����
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98g���þ�����ƽ������Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����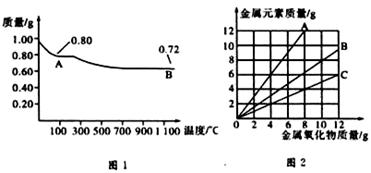
| A��ͼ1�У�A��B�Ĺ�������0.01 mol���ӷ�����ת�� |
| B��ͼ1���������й�����0.26 gˮ |
| C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A |
| D��ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO |
A
���������������0.98 gCu(OH)2��֪�����ʵ���Ϊ0.01 mol����ȫ������CuO��������Ϊ0.01 mol��80 g?mol-1��0.8g������A����CuO����ȫ������Cu2O��������Ϊ0.005 mol��144 g?mol-1��0.72g������B����Cu2O����D����ȷ��A��B�ķ�Ӧ������ͭ�ֽ�����������ͭ�����������ݻ�ѧ����ʽCu(OH)2 CuO+H2O��4CuO
CuO+H2O��4CuO 2Cu2O+O2����֪��0.26 g��ˮ�������������ͣ���B����CuO����������CuԪ�ص�������ϵ����CuO������Ϊ10g���㣩Ϊ�� CuO��Cu
2Cu2O+O2����֪��0.26 g��ˮ�������������ͣ���B����CuO����������CuԪ�ص�������ϵ����CuO������Ϊ10g���㣩Ϊ�� CuO��Cu
80 64
10g 8g
�۲�ͼ2��֪��B���߷�������������ϵ����ʾ����CuO����A���ϵ��κ�һ�㶼��ʾ���������������С��������������Ԫ�ص���������C��������ͭ�����ʵ�����0.01mol������ݷ�Ӧʽ4CuO 2Cu2O+O2����֪��A��B�Ĺ�������0.01 mol���ӷ�����ת�ƣ���A��ȷ����ѡA��
2Cu2O+O2����֪��A��B�Ĺ�������0.01 mol���ӷ�����ת�ƣ���A��ȷ����ѡA��
���㣺����������ͭ�ֽ���йؼ����Լ�ͼ�����

 ��У����ϵ�д�
��У����ϵ�д�������Һ�е�Na+��50 mL 1 mol��L-1 Na3PO3��Һ��Na+�����ʵ���Ũ����ȵ���
| A��150 mL 3 mol��L-1 NaCl��Һ | B��75 mL 2 mol��L-1 NaNO3��Һ |
| C��150 mL 2 mol��L-1 Na2SO4��Һ | D��75 mL 1 mol��L-1��Na2S��Һ |
NA���������ӵ�����������˵����ȷ����
| A��9 g D2O�к��еĵ�����Ϊ5 NA |
| B��46 g NO2��N2O4��������к���ԭ����Ϊ3 NA |
| C��1 mol C2H6�����й��ۼ�����Ϊ8 NA |
| D��7.1 g C12������NaOH��Һ��Ӧת�Ƶĵ�����Ϊ0.2 NA |
������ͬŨ�ȵ�25mL�����зֱ�����������ȵ�NaHCO3��KHCO3�Ļ�����ò���
�����������±���ʾ�������������ܽ⣩
| ��� | 1 | 2 | 3 |
| m(�����)/g | 4.6 | 7.2 | 7.9 |
| V(CO2)(��״��)/L | 1.12 | 1.68 | 1.68 |
���з���������ȷ����
A������ʵ��1�е����ݿ��Լ���������Ũ��
B���������NaHCO3����������ԼΪ45.7%
C�����ݵ�2��3�����ݿ��Է�������2���еĻ������ȫ��Ӧ
D����������ʵ���Ũ��Ϊ1.5mol.L-1
��NAΪ�����ӵ�������ֵ������˵����ȷ����
| A�����³�ѹ�£�14 g ��N2��CO��ɵĻ�����庬�е�ԭ����ĿΪNA |
| B��78 g ������C="C" ˫������ĿΪ3NA |
| C��1 L 1 mol��L-1��NaClO ��Һ�к���ClO������ĿΪNA |
| D����״���£�6.72 L NO2��ˮ��ַ�Ӧת�Ƶĵ�����ĿΪ0.1NA |
����˵����ȷ����
| A��ʹ����Ͳ��ȡһ�������Ũ��������һ�����ʵ���Ũ�ȵ�ϡ���ᣬ��Ũ����ת�����ձ�����������ˮϴ����Ͳ������ϴ��Һһ��ת�����ձ� |
| B������ʹ��480ml 0.1mol/L����ͭ��Һ����ʹ������ƿ������Һ��Ҫ 7.68g����ͭ���� |
| C��ʹ��������ƽ��������ҩƷʱ����������ܻᵼ��������ҺŨ��ƫ�� |
| D������ƿ��ʹ��ǰ���ô�����Һ��ϴ |
�������ʵ����Ľ���Na��Mg��Al�ֱ���100 mL 2 mol/L�����ᷴӦ��ʵ����������������V(���ۺ�Ϊ��״��)��ʱ��t�Ĺ�ϵ��ͼ��ʾ��������˵���������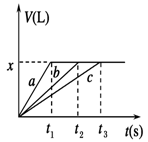
| A��x��2.24 |
| B���Ƶ����ʵ���Ϊ0.2 mol |
| C����Ӧʱ��Na��Mg��Al������ |
| D������bΪMg�����ᷴӦ��ͼ�� |
��50 mL NaOH��Һ����ͨ��һ������CO2(������Һ�������)�����ȡ����Һ10 mL������ϡ����100 mL�������ϡ�ͺ����Һ����μ���0.1 mol��L��1�����ᣬ����CO2��������(��״����)�������������������ϵ��ͼ�����з���������ǣ� ��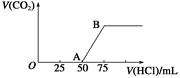
| A��OA����������Ӧ�����ӷ���ʽ�� H����OH��=H2O��CO32����H��=HCO3- |
| B��NaOH������CO2��������Һ������ΪNaOH��Na2CO3�������ʵ���Ũ��֮��Ϊ1��1�� |
| C��������CO2���(��״����)Ϊ0.056 L |
| D��ԭNaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol��L��1 |
���й������ʵ�����Ħ����������������ȷ���ǣ� ��
| A��0.012 kg12C�к���Լ6.02��1023��̼ԭ�� |
| B��1 mol H2O���2 mol���1 mol�� |
| C���������Ƶ�Ħ��������40 g |
| D��2 molˮ��Ħ��������1 molˮ��Ħ��������2�� |