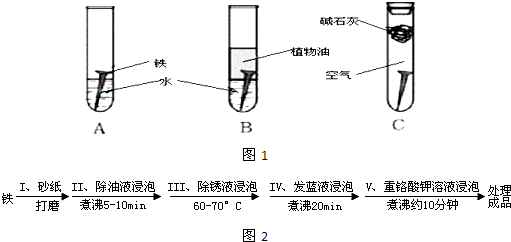
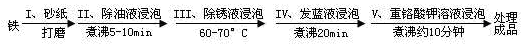��Ŀ����
�������������ǽ�������Ʒ����ijЩ�����Ե���Һ�У��ڸ����ı����γ�һ�������������ļ������̣�����һ�ְ취�ǽ�������Ʒ�����������ƺ�Ũ�������ƵĻ����Һ�м��ȵ�130�淴Ӧ������̿��������»�ѧ����ʽ��ʾ��
��3Fe+NaNO2+5NaOH=3Na2FeO2+H2O+NH3����
��6Na2FeO2+NaNO2+5H2O=3Na2Fe2O4+NH3��+7NaOH��
��Na2FeO2+Na2Fe2O4+2H2O=Fe3O4+4NaOH��
����˵������ȷ���ǣ�������
��3Fe+NaNO2+5NaOH=3Na2FeO2+H2O+NH3����
��6Na2FeO2+NaNO2+5H2O=3Na2Fe2O4+NH3��+7NaOH��
��Na2FeO2+Na2Fe2O4+2H2O=Fe3O4+4NaOH��
����˵������ȷ���ǣ�������
������A���������ɹ����в���������������
B������NԪ�صĻ��ϼ۱仯��������
C������FeԪ�صĻ��ϼ۱仯������ת�Ƶĵ�������
D����Ӧ����û��Ԫ�صĻ��ϼ۱仯��
B������NԪ�صĻ��ϼ۱仯��������
C������FeԪ�صĻ��ϼ۱仯������ת�Ƶĵ�������
D����Ӧ����û��Ԫ�صĻ��ϼ۱仯��
����⣺A����Ӧ���̢٢��ж����ɰ������������д̼�����ζ�����壬��������Ⱦ����A��ȷ��
B����Ӧ����NԪ�صĻ��ϼ���+3�۽���Ϊ-3�ۣ���Ӧ���е���������NaNO2����B��ȷ��
C��n��Fe��=
=0.3mol���ɢ٢ڢۿ�֪��ʧȥ����Ϊ0.2mol����3-0��+0.1mol����2-0��=0.8mol���ӣ���C��ȷ��
D����Ӧ����û��Ԫ�صĻ��ϼ۱仯����Ӧ�۲�����������ԭ��Ӧ����D����
��ѡD��
B����Ӧ����NԪ�صĻ��ϼ���+3�۽���Ϊ-3�ۣ���Ӧ���е���������NaNO2����B��ȷ��
C��n��Fe��=
| 16.8g |
| 56g/mol |
D����Ӧ����û��Ԫ�صĻ��ϼ۱仯����Ӧ�۲�����������ԭ��Ӧ����D����
��ѡD��
���������⿼��������ԭ��Ӧ����ȷԪ�صĻ��ϼ۱仯�ǽ����Ĺؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 ��2011?����ģ�⣩����ͭ������������ʹ�õĽ��������ǵĵ��ʼ�������Ӧ�÷dz��㷺��
��2011?����ģ�⣩����ͭ������������ʹ�õĽ��������ǵĵ��ʼ�������Ӧ�÷dz��㷺��